Influence of Dopant Concentration and Annealing on Binary and Ternary Polymer Blends for Active Materials in OLEDs
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Optical Properties
3.2. Correlation of Absorption and Photoluminescence for Förster Resonance Energy Transfer (FRET)
3.3. Photoluminescence of the Binary and Ternary Thin Films
3.4. Electroluminescence of the Binary and Ternary OLEDs
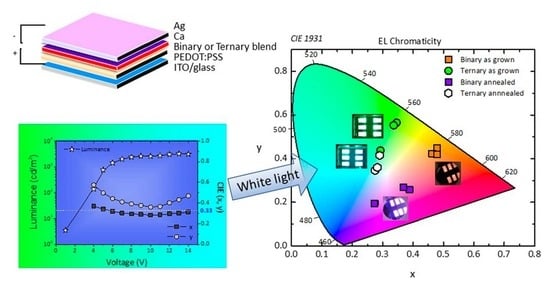
3.5. Chromaticity and Electrical Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- D’Andrade, B.W.; Forrest, S.R. White organic light-emitting devices for solid-state lighting. Adv. Mater. 2004, 16, 1585–1595. [Google Scholar] [CrossRef]
- Reineke, S.; Thomschke, M.; Lussem, B.; Leo, K. White organic light-emitting diodes: Status and perspective. Rev. Mod. Phys. 2013, 85, 1245–1293. [Google Scholar] [CrossRef] [Green Version]
- Karzazi, Y. Organic light emitting diodes: Devices and applications. Environ. Sci. 2014, 5, 1–12. [Google Scholar]
- Aleksandrova, M. Specifics and challenges to flexible organic light-emitting devices. Adv. Mater. Sci. Eng. 2016, 2016, 4081697. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhang, H.; Zhang, B.; Xie, Z.; Wong, W. Towards high-power-efficiency solution-processed OLEDs: Material and device perspectives. Mater. Sci. Eng. R 2020, 140, 100547. [Google Scholar] [CrossRef]
- Yang, H.; Xie, W.; Zhao, Y.; Hou, J.; Liu, S. High efficiency small molecule white organic light-emitting devices with a multilayer structure. Solid State Commun. 2006, 139, 468–472. [Google Scholar] [CrossRef]
- Zhang, S.; Yue, S.; Wu, Q.; Zhang, Z.; Chen, Y.; Wang, X.; Liu, Z.; Xie, G.; Xue, Q.; Qu, D.; et al. Color stable multilayer all phosphor white organic light-emitting diodes with excellent color quality. Org. Electron. 2013, 14, 2014–2022. [Google Scholar] [CrossRef]
- Gioti, M.; Kokkinos, D.; Chaidou, C.I.; Laskarakis, A.; Andreopoulou, A.K.; Kallitsis, J.K.; Logothetidis, S. A comprehensive study of the optical properties of emitting polymers for efficient flexible OLED devices. Phys. Status Solidi A 2016, 213, 2947–2953. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Y.; Tsuboi, T.; Xu, H.; Wua, Y.; Zhang, Z.; Miao, Y.; Hao, Y.; Liu, X.; Xu, B.; et al. Energy transfer in polyfluorene copolymer used for white-light organic light emitting device. Org. Electron. 2013, 14, 827–838. [Google Scholar] [CrossRef]
- Liu, J.; Guo, X.; Bu, L.; Xie, Z.; Cheng, Y.; Wang, Y.G.L.; Jing, X.; Wang, F. White Electroluminescence from a Single-Polymer System with Simultaneous Two-Color Emission: Polyfluorene Blue Host and Side-Chain-Located Orange Dopant. Adv. Funct. Mater. 2007, 17, 1917–1925. [Google Scholar] [CrossRef]
- Tselekidou, D.; Papadopoulos, K.; Kyriazopoulos, V.; Andreopoulou, A.K.; Andrikopoulos, K.; Kallitsis, J.K.; Laskaraki, A.; Logothetidis, S.; Gioti, M. Photophysical and electro-optical properties of copolymers bearing blue and red chromophores for single-layer white OLEDs. Nanomaterials 2021, 11, 2629. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, W.; Wang, B.; He, Y.; Miao, T.; Lü, X.; Fu., G. Single-component white polymer light-emitting diode (WPLED) based on a binary tris-pyrazolonate-Sm-complex. J. Lumin. 2020, 221, 117054. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A. Dual Förster resonance energy transfer in ternary PFO/MEH-PPV/F7GA hybrid thin films for white organic light-emitting diodes. Dye. Pigment. 2021, 186, 109011. [Google Scholar] [CrossRef]
- Franchello, F.; de Menezes, L.C.W.; Renzi, W.; Laureto, E.; Turchetti, D.A.; Akcelrud, L.C.; Ferreira De Deus, J.; Duarte, J.L. Achieving White Emission from Solution Processable Blends of Polyvinylene Derivative Guests into a Polyfluorene Matrix. J. Electron. Mater. 2019, 48, 5980–5987. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A.; AlSalhi, M.S.; Jumali, M.H.H.; Fatehmulla, A.; Qaid, S.M.H.; Mujamammi, W.M.; Ghaithan, H.M. Conjugated Polymers-Based Ternary Hybrid toward Unique Photophysical Properties. Molecules 2022, 27, 7011. [Google Scholar] [CrossRef]
- Guzelturk, B.; Demir, H.V. Near-field energy transfer using Nanoemitters for optoelectronics. Adv. Funct. Mater. 2016, 26, 8158–8177. [Google Scholar] [CrossRef]
- Förster, T. 10th Spiers Memorial Lecture. Transfer mechanisms of electronic excitation. Discuss Faraday Soc. 1959, 27, 7–17. [Google Scholar] [CrossRef]
- Shaheen, S.; Kippelen, B.; Peyghambarian, N.; Wang, J.F.; Anderson, J.; Mash, E.A.; Lee, P.A.; Armstrong, N.R. Energy and charge transfer in organic light-emitting diodes: A soluble quinacridone study. J. Appl. Phys. 1999, 85, 7939–7945. [Google Scholar] [CrossRef]
- Mattoussi, H.; Murata, H.; Merritt, C.D.; Iizumi, Y.; Kido, J.; Kafafi, Z.H. Photoluminescence quantum yield of pure and molecularly doped organic solid films. J. Appl. Phys. 1999, 86, 2642–2650. [Google Scholar] [CrossRef]
- Sun, Y.; Giebink, N.C.; Kanno, H.; Ma, B.; Thompson, M.E.; Forrest, S.R. Management of singlet and triplet excitons for efficient white organic light-emitting devices. Nature 2006, 440, 908–912. [Google Scholar] [CrossRef] [Green Version]
- Kim, N.H.; Kim, Y.H.; Yoon, J.A.; Lee, S.Y.; Ryu, D.H.; Wood, R.; Moon, C.B.; Kim, W.Y. Color optimization of single emissive white OLEDs via energy transfer between RGB fluorescent dopants. J. Lumin. 2013, 143, 723–728. [Google Scholar] [CrossRef]
- Kwon, K.; Lee, Y.K.; Park, T.J.; Uchiike, H.; Kwon, J.H.; Jang, J.; Jin, J.K.; Shin, D.C.; You, H. High-Efficiency White Polymer Light-Emitting Diodes Based on Blended RGB Polymers. Mol. Cryst. Liq. Cryst. 2006, 458, 263–272. [Google Scholar] [CrossRef]
- Gioti, M.; Foris, V.; Kyriazopoulos, V.; Mekeridis, E.; Laskarakis, A.; Logothetidis, S. Optical and electrical characterization of blended active materials for white OLEDs (WOLEDs). Mater. Today Proc. 2021, 37, A32–A38. [Google Scholar] [CrossRef]
- Farahzadi, A.; Beigmohamadi, M.; Niyamakom, P.; Kremers, S.; Meyer, N.; Heuken, M.; Wuttig, M. Characterization of amorphous organic thin films, determination of precise model for spectroscopic ellipsometry measurements. Appl. Surf. Sci. 2010, 256, 6612–6617. [Google Scholar] [CrossRef]
- Jellison, G.E.; Modine, F.A. Parameterization of the optical functions of amorphous materials in the interband region. Appl. Phys. Lett. 1996, 69, 371–373. [Google Scholar] [CrossRef]
- Azzam, R.; Bashara, N. Ellipsometry and Polarized Light; North-Holland Pub.: Amsterdam, The Netherlands, 1977; ISBN 0720406943. [Google Scholar]
- Kim, C.C.; Garland, J.W.; Raccah, P.M. Modeling the optical dielectric function of semiconductors: Extension of the critical-point parabolic-band approximation. Phys. Rev. B 1992, 45, 11749–11767. [Google Scholar] [CrossRef]
- Gioti, M. Optical, photophysical, and electrooptical studies on slot-die polyfluorene-based flexible OLED devices. Opt. Mater. Express 2020, 11, 1442–1456. [Google Scholar] [CrossRef]
- Chen, H.; Su, A.C.; Su, C.H.; Chen, S.A. Crystalline Forms and Emission Behavior of Poly(9, 9-di-n-octyl-2, 7-fluorene). Macromolecules 2005, 38, 379–385. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How to correctly determine the band gap energy of modified semiconductor photocatalysts based on UV–vis spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Charas, A.; Morgado, J.; Martinho, J.M.G.; Fedorov, A.; Alca, L. Excitation energy transfer and spatial exciton confinement in polyfluorene blends for application in light-emitting diodes. J. Mater. Chem. 2002, 23, 3523–3527. [Google Scholar] [CrossRef]
- Kim, D.; Lee, T.; Lee, Y.; Watanabe, T. Experimental verification of Förster energy transfer and quantum resonance between semiconductor quantum dots. Curr. Appl. Phys. 2017, 18, S14–S20. [Google Scholar] [CrossRef]
- Davesnne, C.; Zaini, A.; Labbě, C.; Frilay, C.; Porter, X. Energy transfer mechanism between terbium and europium ions zinc oxide and zinc silicates thin films. Thin Solid Film. 2014, 553, 33–37. [Google Scholar] [CrossRef]
- Kiisk, V.; Tamm, A.; Utt, K.; Kozlova, J.; Mändar, H.; Puust, L.; Aarik, J.; Sildos, I. Photoluminescence of atomic layer deposited ZrO2:Dy3+ thin films. Thin Solid Film. 2015, 583, 70–75. [Google Scholar] [CrossRef]
- Murphy, C.B.; Zhang, Y.; Troxler, T.; Ferry, V.; Martin, J.J.; Jones, W.E. Probing Förster and Dexter energy-transfer mechanisms in fluorescent conjugated polymer Chemosensors. J. Phys. Chem. B 2004, 108, 1537–1543. [Google Scholar] [CrossRef]
- Ling, Q.; Yang, M.; Zhang, W.; Lin, H.; Yu, G.; Bai, F. PL and EL properties of a novel Eu-containing copolymer. Thin Solid Film. 2002, 417, 127–131. [Google Scholar] [CrossRef]
- Wong, K.F.; Bagchi, B.; Rossky, P.J. Distance and orientation dependence of excitation transfer rates in conjugated systems:beyond the Förster theory. J. Phys. Chem. A 2004, 108, 5752–5763. [Google Scholar] [CrossRef]
- Matvienko, O.O.; Savin, Y.N.; Kryzhanovska, A.S.; Vovk, O.M.; Dobrotvorska, M.V.; Pogorelova, N.V.; Vashchenko, V.V. Dispersion and aggregation of quantum dots in polymer-inorganic hybrid films. Thin Solid Film. 2013, 537, 226–230. [Google Scholar] [CrossRef]
- Al-Asbahi, B.A.; Alsalhi, M.S.; Al-Dwayyan, A.S.; Haji Jumali, M.H. Förster-type energy transfer mechanism in PF2/6 to MEH-PPV conjugated polymers. J. Lumin. 2012, 132, 386–390. [Google Scholar] [CrossRef]
- Stryer, L. Fluorescence energy transfer as a spectroscopic ruler. Annu. Rev. Biochem. 1978, 47, 819–846. [Google Scholar] [CrossRef]
- Tovstun, S.A.; Martyanova, E.G.; Brichkin, S.B.; Spirin, M.G.; Gak, V.Y.; Kozlov, A.V.; Razumov, V.F. Förster electronic excitation energy transfer upon adsorption of meso-tetra(3-pyridyl)porphyrin on InP@ZnS colloidal quantum dots. J. Lumin. 2018, 200, 151–157. [Google Scholar] [CrossRef]
- Chen, X.; Wan, H.; Li, H.; Cheng, F.; Ding, J.; Yao, B.; Xie, Z.; Wang, L.; Zhang, J. Influence of thermal annealing temperature on electro-optical properties of polyoctylfluorene thin film: Enhancement of luminescence by self-doping effect of low-content a phase crystallites. Polymer 2012, 53, 3827–3832. [Google Scholar] [CrossRef]
- Chew, K.W.; Rahim, N.A.A.; Teh, P.L.; Hisam, N.S.A.; Alias, S.S. Thermal Degradation of Photoluminescence Poly(9, 9-dioctylfluorene) Solvent-Tuned Aggregate Films. Polymers 2022, 14, 1615. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.H.; Liu, C.Y.; Chang, C.H.; Chen, S.A. Self-Dopant Formation in Poly(9, 9-di-n-octylfluorene) Via a Dipping Method for Efficient and Stable Pure-Blue Electroluminescence. Adv. Mater. (Weinh. Ger.) 2007, 19, 2574–2579. [Google Scholar] [CrossRef]
- Niu, X.; Zhang, B.; Xie, Z.; Cheng, Y.; Wang, L. Balanced charge transport and enhanced white electroluminescence from a single white emissive polymer via thermal annealing. Appl. Phys. Lett. 2010, 96, 073303. [Google Scholar] [CrossRef]
- Donley, C.L.; Zaumseil, J.; Andreasen, J.W.; Nielsen, M.M.; Sirringhaus, H.; Friend, R.H.; Kim, J.S. Effects of packing structure on the optoelectronic and charge transport properties in poly(9, 9-di-n-octylfluorenealt-benzothiadiazole). J. Amer. Chem. Soc. 2005, 127, 12890–12899. [Google Scholar] [CrossRef]
- Kreouzis, T.; Poplavskyy, D.; Tuladhar, S.M.; Campoy-Quiles, M.; Nelson, J.; Campbell, A.J.; Bradley, D.D.C. Temperature and field dependence of hole mobility in poly(9, 9-dioctylfluorene). Phys. Rev. B 2006, 73, 235201–235215. [Google Scholar] [CrossRef]
- Voigt, M.; Chappell, J.; Rowson, T.; Cadby, A.; Geoghegan, M.; Jones, R.A.L.; Lidzey, D.G. The interplay between the optical and electronic properties of light-emitting-diode applicable conjugated polymer blends and their phase-separated morphology. Org. Electron. 2005, 6, 35–45. [Google Scholar] [CrossRef]








| Blend Type | Blend Code | |||||
|---|---|---|---|---|---|---|
| Binary#1 | Binary#2 | Binary#3 | Ternary#1 | Ternary#2 | Ternary#3 | |
| PFO:SPR | 95:5 | 97.5:2.5 | 99:1 | |||
| PFO:F8BT:SPR | 97:2.5:0.5 | 98:1.5:0.5 | 99:0.5:0.5 | |||
| Material/Blend Type | Thickness d (nm) | (eV) | (eV) | (eV) | |
|---|---|---|---|---|---|
| SPR | as-grown | 73.6 | 1.53 | 2.54 | 1.01 |
| annealed | 72.2 | 1.56 | 2.54 | 0.98 | |
| F8BT | as-grown | 62.7 | 2.36 | 2.55 | 0.19 |
| annealed | 61.7 | 2.36 | 2.55 | 0.19 | |
| PFO | as-grown | 46.9 | 2.87 | 3.06 | 0.19 |
| annealed | 48.0 | 2.88 | 3.07 | 0.19 | |
| Binary#1 | as-grown | 48.3 | 2.88 | 3.07 | 0.19 |
| annealed | 56.5 | 2.79 | 3.04 | 0.25 | |
| Ternary#1 | as-grown | 46.3 | 2.88 | 3.05 | 0.17 |
| annealed | 38.5 | 2.76 | 2.97 | 0.21 | |
| Blend Type/ Treatment | Turn-on Voltage (V) at 10 cd/m2 | V at Max Luminance (V) | Max Luminance (cd/m2) | CRI | x | y | |
|---|---|---|---|---|---|---|---|
| Binary#1 | as-grown | 5.5 | 12 | 1033 | 94 | 0.474 | 0.436 |
| annealed | 6.0 | 12 | 830 | 57 | 0.388 | 0.259 | |
| Binary#2 | as-grown | 3.5 | 12 | 1038 | 94 | 0.460 | 0.424 |
| annealed | 6.5 | 12 | 721 | 64 | 0.369 | 0.269 | |
| Binary#3 | as-grown | 6.0 | 12 | 561 | 95 | 0.479 | 0.419 |
| annealed | 6.5 | 12 | 401 | 50 | 0.273 | 0.194 | |
| Ternary#1 | as-grown | 3.0 | 13 | 7474 | 44 | 0.339 | 0.557 |
| annealed | 4.0 | 11 | 7849 | 62 | 0.290 | 0.415 | |
| Ternary#2 | as-grown | 3.5 | 14 | 9838 | 49 | 0.345 | 0.560 |
| annealed | 3.0 | 12 | 3614 | 72 | 0.275 | 0.346 | |
| Ternary#3 | as-grown | 3.5 | 14 | 3321 | 74 | 0.250 | 0.300 |
| annealed | 2.0 | 14 | 3184 | 70 | 0.282 | 0.360 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gioti, M.; Tselekidou, D.; Foris, V.; Kyriazopoulos, V.; Papadopoulos, K.; Kassavetis, S.; Logothetidis, S. Influence of Dopant Concentration and Annealing on Binary and Ternary Polymer Blends for Active Materials in OLEDs. Nanomaterials 2022, 12, 4099. https://doi.org/10.3390/nano12224099
Gioti M, Tselekidou D, Foris V, Kyriazopoulos V, Papadopoulos K, Kassavetis S, Logothetidis S. Influence of Dopant Concentration and Annealing on Binary and Ternary Polymer Blends for Active Materials in OLEDs. Nanomaterials. 2022; 12(22):4099. https://doi.org/10.3390/nano12224099
Chicago/Turabian StyleGioti, Maria, Despoina Tselekidou, Vasileios Foris, Vasileios Kyriazopoulos, Kyparisis Papadopoulos, Spyros Kassavetis, and Stergios Logothetidis. 2022. "Influence of Dopant Concentration and Annealing on Binary and Ternary Polymer Blends for Active Materials in OLEDs" Nanomaterials 12, no. 22: 4099. https://doi.org/10.3390/nano12224099
APA StyleGioti, M., Tselekidou, D., Foris, V., Kyriazopoulos, V., Papadopoulos, K., Kassavetis, S., & Logothetidis, S. (2022). Influence of Dopant Concentration and Annealing on Binary and Ternary Polymer Blends for Active Materials in OLEDs. Nanomaterials, 12(22), 4099. https://doi.org/10.3390/nano12224099