Carbon-Supported PdCu Alloy as Extraordinary Electrocatalysts for Methanol Electrooxidation in Alkaline Direct Methanol Fuel Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of Catalysts
2.3. Physical Property Characterization
2.4. Electrochemical and Physical Characterization
3. Results
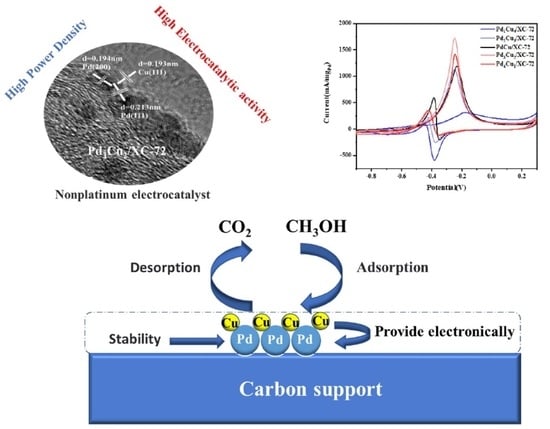
3.1. Physical Characterization of PdxCuy/XC-72
3.2. Electrochemical Tests for MOR (Three-Electrode Cell)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fu, X.; Wan, C.; Huang, Y.; Duan, X. Noble Metal Based Electrocatalysts for Alcohol Oxidation Reactions in Alkaline Media. Adv. Funct. Mater. 2022, 32, 2106401. [Google Scholar] [CrossRef]
- Bianchini, C.; Shen, P.K. Palladium-Based Electrocatalysts for Alcohol Oxidation in Half Cells and in Direct Alcohol Fuel Cells. Chem. Rev. 2009, 109, 4183–4206. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Zhang, X.; Sun, H.; Wang, S.; Sun, G. Recent Advances in Multi-Scale Design and Construction of Materials for Direct Methanol Fuel Cells. Nano Energy 2019, 65, 104048. [Google Scholar] [CrossRef]
- Tong, Y.Y.; Gu, C.D.; Zhang, J.L.; Huang, M.L.; Tang, H.; Wang, X.L.; Tu, J.P. Three-Dimensional Astrocyte-Network Ni–P–O Compound with Superior Electrocatalytic Activity and Stability for Methanol Oxidation in Alkaline Environments. J. Mater. Chem. A 2015, 3, 4669–4678. [Google Scholar] [CrossRef]
- de Sá, M.H.; Pinto, A.M.F.R.; Oliveira, V.B. Passive Direct Methanol Fuel Cells as a Sustainable Alternative to Batteries in Hearing Aid Devices—An Overview. Int. J. Hydrogen Energy 2022, 47, 16552–16567. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, Z.; Jiang, J.; Wang, J.; Song, X.; He, Q.; Ding, W.; Wei, Z. Author Correction: Lattice-Confined Ru Clusters with High CO Tolerance and Activity for the Hydrogen Oxidation Reaction. Nat. Catal. 2021, 4, 341. [Google Scholar] [CrossRef]
- Wan, X.; Liu, X.; Li, Y.; Yu, R.; Zheng, L.; Yan, W.; Wang, H.; Xu, M.; Shui, J. Fe–N–C Electrocatalyst with Dense Active Sites and Efficient Mass Transport for High-Performance Proton Exchange Membrane Fuel Cells. Nat. Catal. 2019, 2, 259–268. [Google Scholar] [CrossRef]
- Anson, C.W.; Stahl, S.S. Mediated Fuel Cells: Soluble Redox Mediators and Their Applications to Electrochemical Reduction of O2 and Oxidation of H2, Alcohols, Biomass, and Complex Fuels. Chem. Rev. 2020, 120, 3749–3786. [Google Scholar] [CrossRef]
- Luo, M.; Zhao, Z.; Zhang, Y.; Sun, Y.; Xing, Y.; Lv, F.; Yang, Y.; Zhang, X.; Hwang, S.; Qin, Y.; et al. PdMo Bimetallene for Oxygen Reduction Catalysis. Nature 2019, 574, 81–85. [Google Scholar] [CrossRef]
- Zhu, J.; Xia, L.; Yu, R.; Lu, R.; Li, J.; He, R.; Wu, Y.; Zhang, W.; Hong, X.; Chen, W.; et al. Ultrahigh Stable Methanol Oxidation Enabled by a High Hydroxyl Concentration on Pt Clusters/MXene Interfaces. J. Am. Chem. Soc. 2022, 144, 15529–15538. [Google Scholar] [CrossRef]
- Zuo, Y.; Sheng, W.; Tao, W.; Li, Z. Direct Methanol Fuel Cells System–A Review of Dual-Role Electrocatalysts for Oxygen Reduction and Methanol Oxidation. J. Mater. Sci. Technol. 2022, 114, 29–41. [Google Scholar] [CrossRef]
- Ali, A.; Shen, P.K. Recent Advances in Graphene-Based Platinum and Palladium Electrocatalysts for the Methanol Oxidation Reaction. J. Mater. Chem. A 2019, 7, 22189–22217. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, L.; Liao, S.; Zeng, J. Pt∧Ru/C Catalysts Synthesized by a Two-Stage Polyol Reduction Process for Methanol Oxidation Reaction. J. Power Sources 2011, 196, 10570–10575. [Google Scholar] [CrossRef]
- Lee, E.; Kim, S.; Jang, J.-H.; Park, H.-U.; Matin, M.A.; Kim, Y.-T.; Kwon, Y.-U. Effects of Particle Proximity and Composition of Pt–M (M = Mn, Fe, Co) Nanoparticles on Electrocatalysis in Methanol Oxidation Reaction. J. Power Sources 2015, 294, 75–81. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Li, G.; Wang, D.; Wang, S.; Zhao, X. Low-Temperature N-Anchored Ordered Pt3Co Intermetallic Nanoparticles as Electrocatalysts for Methanol Oxidation Reaction. Nanoscale 2022, 14, 14199–14211. [Google Scholar] [CrossRef] [PubMed]
- Fan, A.; Qin, C.; Zhao, R.; Sun, H.; Sun, H.; Dai, X.; Ye, J.-Y.; Sun, S.-G.; Lu, Y.; Zhang, X. Phosphorus-Doping-Tuned PtNi Concave Nanocubes with High-Index Facets for Enhanced Methanol Oxidation Reaction. Nano Res. 2022, 15, 6961–6968. [Google Scholar] [CrossRef]
- Chen, G.; Yang, X.; Xie, Z.; Zhao, F.; Zhou, Z.; Yuan, Q. Hollow PtCu Octahedral Nanoalloys: Efficient Bifunctional Electrocatalysts towards Oxygen Reduction Reaction and Methanol Oxidation Reaction by Regulating near-Surface Composition. J. Colloid Interface Sci. 2020, 562, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lei, Z.; Zeng, T.; Wang, L.; Cheng, N.; Tan, Y.; Mu, S. Structurally Ordered PtSn Intermetallic Nanoparticles Supported on ATO for Efficient Methanol Oxidation Reaction. Nanoscale 2019, 11, 19895–19902. [Google Scholar] [CrossRef]
- Qiao, M.; Wu, H.; Meng, F.; Zhuang, Z.; Wang, J. Defect-Rich, Highly Porous PtAg Nanoflowers with Superior Anti-Poisoning Ability for Efficient Methanol Oxidation Reaction. Small 2022, 18, 2106643. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xia, T.; Wang, S.; Yang, G.; Dong, B.; Wang, C.; Ma, Q.; Sun, Y.; Wang, R. Oriented-Assembly of Hollow FePt Nanochains with Tunable Catalytic and Magnetic Properties. Nanoscale 2016, 8, 11432–11440. [Google Scholar] [CrossRef]
- Fan, H.; Cheng, M.; Wang, Z.; Wang, R. Layer-Controlled Pt-Ni Porous Nanobowls with Enhanced Electrocatalytic Performance. Nano Res. 2017, 10, 187–198. [Google Scholar] [CrossRef]
- Zhou, M.; Guo, J.; Fang, J. Nanoscale Design of Pd-Based Electrocatalysts for Oxygen Reduction Reaction Enhancement in Alkaline Media. Small Struct. 2022, 3, 2100188. [Google Scholar] [CrossRef]
- Shih, Z.-Y.; Wang, C.-W.; Xu, G.; Chang, H.-T. Porous Palladium Copper Nanoparticles for the Electrocatalytic Oxidation of Methanol in Direct Methanol Fuel Cells. J. Mater. Chem. A 2013, 1, 4773. [Google Scholar] [CrossRef]
- Lei, H.; Zhang, Q. In Situ Electrochemical Redox Tuning of Pd-Co Hybrid Electrocatalysts for High-Performance Methanol Oxidation: Strong Metal-Support Interaction. J. Colloid Interface Sci. 2021, 588, 476–484. [Google Scholar] [CrossRef]
- Lao, X.; Sun, T.; Zhang, X.; Pang, M.; Fu, A.; Guo, P. Controllable Lattice Expansion of Monodisperse Face-Centered Cubic Pd–Ag Nanoparticles for C1 and C2 Alcohol Oxidation: The Role of Core–Sheath Lattice Mismatch. ACS Sustain. Chem. Eng. 2022, 10, 6843–6852. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, S.; Sun, X.; Sun, S. Synthesis of Ultrathin FePtPd Nanowires and Their Use as Catalysts for Methanol Oxidation Reaction. J. Am. Chem. Soc. 2011, 133, 15354–15357. [Google Scholar] [CrossRef]
- Chen, S.; Liu, N.; Zhong, J.; Yang, R.; Yan, B.; Gan, L.; Yu, P.; Gui, X.; Yang, H.; Yu, D.; et al. Engineering Support and Distribution of Palladium and Tin on MXene with the Modulation D-Band Center for CO-resilient Methanol Oxidation. Angew. Chem. Int. Ed. 2022, 61, e202209693. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Bai, Y.; Jiang, Z.; Fang, T. Design the PdCu/TaN C Electrocatalyst with Core-Shell Structure Having High Efficiency for Methanol and Formic Acid Oxidation Reactions. Electrochim. Acta 2021, 383, 138365. [Google Scholar] [CrossRef]
- Saleem, F.; Zhang, Z.; Cui, X.; Gong, Y.; Chen, B.; Lai, Z.; Yun, Q.; Gu, L.; Zhang, H. Elemental Segregation in Multimetallic Core–Shell Nanoplates. J. Am. Chem. Soc. 2019, 141, 14496–14500. [Google Scholar] [CrossRef]
- Hu, S.; Munoz, F.; Noborikawa, J.; Haan, J.; Scudiero, L.; Ha, S. Carbon Supported Pd-Based Bimetallic and Trimetallic Catalyst for Formic Acid Electrochemical Oxidation. Appl. Catal. B Environ. 2016, 180, 758–765. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, B.; Dong, S.; Wang, C.; Feng, A.; Fan, X.; Li, Y. Reduced Graphene Oxide Supported Pd-Cu-Co Trimetallic Catalyst: Synthesis, Characterization and Methanol Electrooxidation Properties. J. Energy Chem. 2019, 29, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Tan, Q.; Shu, C.; Abbott, J.; Zhao, Q.; Liu, L.; Qu, T.; Chen, Y.; Zhu, H.; Liu, Y.; Wu, G. Highly Dispersed Pd-CeO 2 Nanoparticles Supported on N-Doped Core–Shell Structured Mesoporous Carbon for Methanol Oxidation in Alkaline Media. ACS Catal. 2019, 9, 6362–6371. [Google Scholar] [CrossRef]
- Jin, L.; Xu, H.; Chen, C.; Shang, H.; Wang, Y.; Wang, C.; Du, Y. Three-Dimensional PdCuM (M = Ru, Rh, Ir) Trimetallic Alloy Nanosheets for Enhancing Methanol Oxidation Electrocatalysis. ACS Appl. Mater. Interfaces 2019, 11, 42123–42130. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Li, F.; Huang, H.; Yin, S.; Chen, P.; Jin, P.; Chen, Y. Porous Pd-PdO Nanotubes for Methanol Electrooxidation. Adv. Funct. Mater. 2020, 30, 2000534. [Google Scholar] [CrossRef]
- Luo, X.; Liu, C.; Wang, X.; Shao, Q.; Pi, Y.; Zhu, T.; Li, Y.; Huang, X. Spin Regulation on 2D Pd–Fe–Pt Nanomeshes Promotes Fuel Electrooxidations. Nano Lett. 2020, 20, 1967–1973. [Google Scholar] [CrossRef]
- Xue, J.; Hu, Z.; Li, H.; Zhang, Y.; Liu, C.; Li, M.; Yang, Q.; Hu, S. Pd-Sn Alloy Nanoparticles for Electrocatalytic Methanol Oxidation: Phase Evolution from Solid Solution to Intermetallic Compounds. Nano Res. 2022, 15, 8819–8825. [Google Scholar] [CrossRef]








| Sample | ICP Results (wt%) | Theory of Loads (wt%) | Theoretical Metal Molar Ratio (Pd: Cu) | Actual Metal Molar Ratio (Pd: Cu) | ||
|---|---|---|---|---|---|---|
| Pd | Cu | Pd | Cu | |||
| Pd1Cu4/XC-72 | 7.6 | 15.4 | 8.8 | 21.2 | 0.40 | 0.47 |
| Pd2Cu3/XC-72 | 12.4 | 11.1 | 15.8 | 14.2 | 1.07 | 1.07 |
| PdCu/XC-72 | 18.2 | 10.2 | 18.7 | 11.3 | 1.60 | 1.72 |
| Pd3Cu2/XC-72 | 19.7 | 6.9 | 21.4 | 8.6 | 2.40 | 2.74 |
| Pd4Cu1/XC-72 | 25.8 | 3.5 | 26.1 | 3.9 | 6.44 | 7.08 |
| Catalyst | Test Condition | Scan Rate (mV/s) | Mass Activity (mA/mg) | Refs. |
|---|---|---|---|---|
| Pd3Cu2/XC-72 | 1 M KOH + 1 M Methanol | 50 mV/s | 1719 mA/mg | This Work |
| Pd/C | 1 M KOH + 1 M methanol | 50 mV/s | 550 mA/mg | 2019 [32] |
| Solid carbon sphere-supported Pd-CeO2 nanoparticles | 1 M KOH + 1 M methanol | 50 mV/s | 900 mA/mg | 2019 [32] |
| Pd59Cu33 Ru8 NSs | 1 M KOH + 1 M methanol | 50 mV/s | 1660.8 mA/mg | 2019 [33] |
| Pd-PdO PNTs-260 | 1 M KOH + 1 M methanol | 50 mV/s | 1111.3 mA/mg | 2020 [34] |
| Pd59Fe27Pt14 NMs | 1 M KOH + 1 M methanol | 50 mV/s | 1610 mA/mg | 2020 [35] |
| Pd3Sn2 IMC | 1 M KOH + 1 M methanol | 50 mV/s | 1300 mA/mg | 2022 [36] |
| Pd/Se−Ti3C2 | 1 M KOH + 1 M methanol | 50 mV/s | 1046.2 mA/mg | 2022 [27] |
| Pd72Cu14Co14/rGO | 1 M KOH + 1 M methanol | 50 mV/s | 1062 mA/mg | 2019 [31] |
| Pd1Cu4/XC-72 | Pd2Cu3/XC-72 | PdCu/XC-72 | Pd3Cu2/XC-72 | Pd4Cu1/XC-72 | |
|---|---|---|---|---|---|
| Mass activity | 311 mA/mgPd | 1130 mA/mgPd | 1189 mA/mgPd | 1719 mA/mgPd | 1409 mA/mgPd |
| If/Ib | 3.41287 | 4.01877 | 1.999 | 5.307 | 4.785 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, G.; Wang, S.; Li, H.; Guo, P.; Li, Y.; Ji, D.; Zhao, X. Carbon-Supported PdCu Alloy as Extraordinary Electrocatalysts for Methanol Electrooxidation in Alkaline Direct Methanol Fuel Cells. Nanomaterials 2022, 12, 4210. https://doi.org/10.3390/nano12234210
Li G, Wang S, Li H, Guo P, Li Y, Ji D, Zhao X. Carbon-Supported PdCu Alloy as Extraordinary Electrocatalysts for Methanol Electrooxidation in Alkaline Direct Methanol Fuel Cells. Nanomaterials. 2022; 12(23):4210. https://doi.org/10.3390/nano12234210
Chicago/Turabian StyleLi, Guixian, Shoudeng Wang, Hongwei Li, Peng Guo, Yanru Li, Dong Ji, and Xinhong Zhao. 2022. "Carbon-Supported PdCu Alloy as Extraordinary Electrocatalysts for Methanol Electrooxidation in Alkaline Direct Methanol Fuel Cells" Nanomaterials 12, no. 23: 4210. https://doi.org/10.3390/nano12234210
APA StyleLi, G., Wang, S., Li, H., Guo, P., Li, Y., Ji, D., & Zhao, X. (2022). Carbon-Supported PdCu Alloy as Extraordinary Electrocatalysts for Methanol Electrooxidation in Alkaline Direct Methanol Fuel Cells. Nanomaterials, 12(23), 4210. https://doi.org/10.3390/nano12234210


_Hui.png)