Mono-Doped and Co-Doped Nanostructured Hematite for Improved Photoelectrochemical Water Splitting
Abstract
:1. Introduction
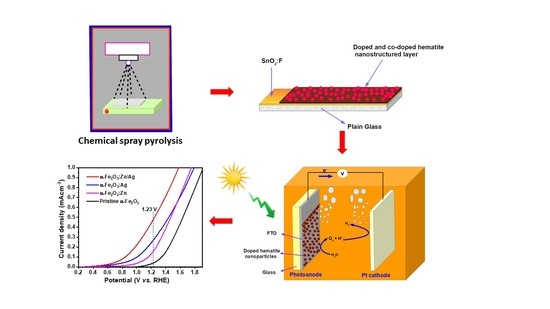
2. Experimental Details
3. Results and Discussion
3.1. X-ray Diffraction
3.2. Morphology of Hematite Nanoparticles
3.3. Optical Properties
3.4. Photocurrent Density Measurements
3.5. Electrochemical Impedance Measurements
3.6. Ultrafast Transient Absorption Spectroscopy
3.7. Global Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meng, J.; Hu, X.; Chen, P.; Coffman, D.; Han, M. The unequal contribution to global energy consumption along the supply chain. J. Environ. Manag. 2020, 268, 110701. [Google Scholar] [CrossRef]
- Qiu, Y.; Leung, S.; Zhang, Q.; Hua, B.; Lin, Q.; Wei, Z.; Tsui, K.; Zhang, Y.; Yang, S.; Fan, Z. Efficient photoelectrochemical water splitting with ultrathin films of hematite on three-dimensional nanophotonic structures. Nano Lett. 2014, 14, 2123–2129. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D.A.; Wang, G.; Ling, Y.; Li, Y.; Zhang, J.Z. Nanostructured hematite: Synthesis, characterization, charge carrier dynamics, and photoelectrochemical properties. Energy Environ. Sci. 2012, 5, 6682–6702. [Google Scholar] [CrossRef]
- Tsege, E.L.; Atabaev, T.S.; Hossain, M.A.; Lee, D.; Kim, H.K.; Hwang, Y.H. Cu-doped flower-like hematite nanostructures for efficient water splitting applications. J. Phys. Chem. Solids 2016, 98, 283–289. [Google Scholar] [CrossRef]
- Wang, J.J.; Hu, Y.; Toth, R.; Fortunato, G.; Braun, A. A facile nonpolar organic solution process of a nanostructured hematite photoanode with high efficiency and stability for water splitting. R. Soc. Chem. 2016, 4, 2821–2825. [Google Scholar] [CrossRef]
- Kumari, S.; Singh, A.P.; Tripathi, C.; Chauhan, D.; Dass, S.; Shrivastav, R.; Gupta, V.; Sreenivas, K.; Satsangi, V.R. Enhanced photoelectrochemical response of Zn-dotted hematite. Int. J. Photoenergy 2007, 2007, 087467. [Google Scholar] [CrossRef] [Green Version]
- Hoyle, R.; Sotomayor, J.; Will, G.; Fitzmaurice, D. Visible-light-induced and long-lived charge separation in a transparent nanostructured semiconductor membrane modified by an adsorbed electron donor and electron acceptor. J. Phys. Chem. B 1997, 101, 10791–10800. [Google Scholar] [CrossRef]
- Garoufalis, C.S.; Poulopoulos, P.; Bouropoulos, N.; Barnasas, A.; Baskoutas, S. Growth and optical properties of α-Fe2O3 thin films: A study of quantum confinement effects by experiment and theory. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 89, 67–71. [Google Scholar] [CrossRef]
- Barroso, M.; Cowan, A.J.; Pendlebury, S.R.; Gratzel, M.; Klug, D.R.; Durrant, J.R. The role of cobalt phosphate in enhancing the photocatalytic activity of α-Fe2O3 toward water oxidation. J. Am. Chem. Soc. 2011, 133, 14868–14871. [Google Scholar] [CrossRef]
- Hisatomi, T.; Dotan, H.; Stefik, M.; Sivula, K.; Rothschild, A.; Graetzel, M.; Mathews, N. Enhancement in the performance of ultrathin hematite photoanode for water splitting by an oxide underlayer. Adv. Mater. 2012, 24, 2699–2702. [Google Scholar] [CrossRef]
- Paradzah, A.T.; Diale, M.; Maabong, K.; Krüger, T.P.J. Use of interfacial layers to prolong hole lifetimes in hematite probed by ultrafast transient absorption spectroscopy. Phys. B Condens. Matter 2018, 535, 138–142. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.; Zhou, J.; Dong, C.L.; Hu, Y.; Tseng, E.N.; Guo, P.; Guo, L.; Mao, S.S. Surface engineered doping of hematite nanorod arrays for improved photoelectrochemical water splitting. Sci. Rep. 2014, 4, 6627. [Google Scholar] [CrossRef]
- Ahn, H.J.; Yoon, K.Y.; Kwak, M.J.; Park, J.; Jang, J.H. Boron doping of metal-doped hematite for reduced surface recombination in water splitting. ACS Catal. 2018, 8, 11932–11939. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, H.; Ma, W.; Chen, C.; Song, W.; Zhao, J. Doping-promoted solar water oxidation on hematite photoanodes. Molecules 2016, 21, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pendlebury, S.R.; Barroso, M.; Cowan, A.J.; Sivula, K.; Tang, J.; Grätzel, M.; Klug, D.; Durrant, J.R. Dynamics of photogenerated holes in nanocrystalline α-Fe2O3 electrodes for water oxidation probed by transient absorption spectroscopy. Chem. Commun. 2011, 47, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Paradzah, A.T.; Maabong, K.D.; Elnour, H.M.; Singh, A.; Diale, M.; Kruger, T.P.J. Identification of Exciton–Exciton Annihilation in Hematite Thin Films. J. Phys. Chem. C 2019, 123, 18676–18684. [Google Scholar] [CrossRef]
- Congolo, S.; Madito, M.J.; Paradzah, A.T.; Harrison, A.J.; Elnour, H.M.A.M.; Krüger, T.P.J.; Diale, M. Reduction of recombination rates due to volume increasing, annealing, and tetraethoxysilicate treatment in hematite thin films. Appl. Nanosci. 2020, 10, 1957–1967. [Google Scholar] [CrossRef]
- Pei, G.X.; Wijten, J.H.; Weckhuysen, B.M. Probing the dynamics of photogenerated holes in doped hematite photoanodes for solar water splitting using transient absorption spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 9806–9811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pendlebury, S.R.; Wang, X.; Le Formal, F.; Cornuz, M.; Kafizas, A.; Tilley, S.D.; Gratzel, M.; Durrant, J.R. Ultrafast charge recombination and trapping in hematite photoanodes under applied bias. J. Am. Chem. Soc. 2014, 136, 9854–9857. [Google Scholar] [CrossRef] [Green Version]
- Nyarige, J.S.; Krüger, T.P.J.; Diale, M. Effects of L-arginine concentration on hematite nanostructures synthesized by spray pyrolysis and chemical bath deposition. Phys. B Condens. Matter 2019, 581, 411924. [Google Scholar] [CrossRef]
- Nyarige, J.S.; Krüger, T.P.J.; Diale, M. Influence of precursor concentration and deposition temperature on the photoactivity of hematite electrodes for water splitting. Mater. Today Commun. 2020, 25, 101459. [Google Scholar] [CrossRef]
- Nyarige, J.S.; Krüger, T.P.J.; Diale, M. Structural and optical properties of hematite and L-arginine/hematite nanostructures prepared by thermal spray pyrolysis. Surfaces Interfaces 2020, 18, 100394. [Google Scholar] [CrossRef]
- Snellenburg, J.J.; Laptenok, S.P.; Seger, R.; Mullen, K.M.; van Stokkum, I.H. Glotaran: A Java-Based Graphical User Interface for the R Package TIMP. J. Stat. Softw. 2012, 49, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Chiam, S.Y.; Kumar, M.H.; Bassi, P.S.; Seng, H.L.; Barber, J.; Wong, L.H. Improving the efficiency of hematite nanorods for photoelectrochemical water splitting by doping with manganese. ACS Appl. Mater. Interfaces 2014, 6, 5852–5859. [Google Scholar]
- Mirbagheri, N.; Wang, D.; Peng, C.; Wang, J.; Huang, Q.; Fan, C.; Ferapontova, E.E. Visible light driven photoelectrochemical water oxidation by Zn-and Ti-doped hematite nanostructures. ACS Catal. 2014, 4, 2006–2015. [Google Scholar] [CrossRef]
- Aragaw, B.A.; Pan, C.J.; Su, W.N.; Chen, H.M.; Rick, J.; Hwang, B.J. Facile one-pot controlled synthesis of Sn and C codoped single crystal TiO2 nanowire arrays for highly efficient photoelectrochemical water splitting. Appl. Catal. B Environ. 2015, 163, 478–486. [Google Scholar] [CrossRef]
- Sivula, K.; Le Formal, F.; Grätzel, M. Solar water splitting: Progress using hematite (α-Fe2O3) photoelectrodes. ChemSusChem 2011, 4, 432–449. [Google Scholar] [CrossRef]
- Souza, F.L.; Lopes, K.P.; Longo, E.; Leite, E.R. The influence of the film thickness of nanostructured α-Fe2O3 on water photooxidation. Phys. Chem. Chem. Phys. 2009, 11, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Tossell, J.A.; Vaughan, D.J.; Johnson, K.H. The electronic structure of rutile, wustite, and hematite from molecular orbital calculations. Am. Mineral. J. Earth Planet. Mater. 1974, 59, 319–334. [Google Scholar]
- Marusak, L.A.; Messier, R.; White, W.B. Optical absorption spectrum of hematite, α-Fe2O3 near IR to UV. J. Phys. Chem. Solids 1980, 41, 981–984. [Google Scholar] [CrossRef]
- Qiu, P.; Yang, H.; Yang, L.; Wang, Q.; Ge, L. Solar water splitting with nanostructured hematite: The role of annealing-temperature. Electrochim. Acta 2018, 266, 431–440. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Zhu, S.S.; Liu, Y.Y.; Zhang, X.; Wang, J.J.; Braun, A. Covalent S-O bonding enables enhanced photoelectrochemical performance of Cu2S/Fe2O3 heterojunction for water splitting. Small 2021, 17, 2100320. [Google Scholar] [CrossRef]
- Zhu, S.S.; Zhang, Y.; Zou, Y.; Guo, S.Y.; Liu, H.; Wang, J.J.; Braun, A. Cu2S/BiVO4 Heterostructure photoanode with extended wavelength range for efficient water splitting. J. Phys. Chem. C 2021, 29, 15890–15898. [Google Scholar] [CrossRef]
- Chen, Y.C.; Kuo, C.L.; Hsu, Y.K. Facile preparation of Zn-doped hematite thin film as photocathode for solar hydrogen generation. J. Alloys Compd. 2018, 768, 810–816. [Google Scholar] [CrossRef]
- Kennedy, J.H.; Frese, K.W., Jr. Flatband Potentials and Donor Densities of Polycrystalline α-Fe2O3 Determined from Mott-Schottky Plots. J. Electrochem. Soc. 1978, 125, 723. [Google Scholar] [CrossRef]
- Li, M.; Deng, J.; Pu, A.; Zhang, P.; Zhang, H.; Gao, J.; Hao, Y.; Zhong, J.; Sun, X. Hydrogen-treated hematite nanostructures with low onset potential for highly efficient solar water oxidation. J. Mater. Chem. A 2014, 2, 6727–6733. [Google Scholar] [CrossRef]
- Xi, L.; Bassi, P.S.; Chiam, S.Y.; Mak, W.F.; Tran, P.D.; Barber, J.; Loo, J.S.C.; Wong, L.H. Surface treatment of hematite photoanodes with zinc acetate for water oxidation. Nanoscale 2012, 4, 4430–4433. [Google Scholar] [CrossRef]
- Gelderman, K.; Lee, L.; Donne, S.W. Flat-band potential of a semiconductor: Using the Mott–Schottky equation. J. Chem. Educ. 2007, 84, 685. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jun, H.; Hong, S.J.; Kim, H.G.; Lee, J.S. Charge transfer in iron oxide photoanode modified with carbon nanotubes for photoelectrochemical water oxidation: An electrochemical impedance study. Int. J. Hydrog. Energy 2011, 36, 9462–9468. [Google Scholar] [CrossRef]
- Yu, Q.; Meng, X.; Wang, T.; Li, P.; Ye, J. Hematite films decorated with nanostructured ferric oxyhydroxide as photoanodes for efficient and stable photoelectrochemical water splitting. Adv. Funct. Mater. 2015, 25, 2686–2692. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jang, J.W.; Youn, D.H.; Kim, J.Y.; Kim, E.S.; Lee, J.S. Graphene–carbon nanotube composite as an effective conducting scaffold to enhance the photoelectrochemical water oxidation activity of a hematite film. RSC Adv. 2012, 2, 9415–9422. [Google Scholar]
- Fitzmorris, B.C.; Patete, J.M.; Smith, J.; Mascorro, X.; Adams, S.; Wong, S.S.; Zhang, J.Z. Ultrafast transient absorption studies of hematite nanoparticles: The effect of particle shape on exciton dynamics. ChemSusChem 2013, 6, 1907–1914. [Google Scholar] [CrossRef]
- Barroso, M.; Mesa, C.A.; Pendlebury, S.R.; Cowan, A.J.; Hisatomi, T.; Sivula, K.; Grätzel, M.; Klug, D.R.; Durrant, J.R. Dynamics of photogenerated holes in surface modified α-Fe2O3 photoanodes for solar water splitting. Proc. Natl. Acad. Sci. USA 2012, 109, 15640–15645. [Google Scholar] [CrossRef] [Green Version]
- Sorenson, S.; Driscoll, E.; Haghighat, S.; Dawlaty, J.M. Ultrafast carrier dynamics in hematite films: The role of photoexcited electrons in the transient optical response. J. Phys. Chem. C 2014, 118, 23621–23626. [Google Scholar] [CrossRef]
- Cherepy, N.J.; Liston, D.B.; Lovejoy, J.A.; Deng, H.; Zhang, J.Z. Ultrafast studies of photoexcited electron dynamics in α-Fe2O3 semiconductor nanoparticles. J. Phys. Chem. B 1998, 102, 770–776. [Google Scholar] [CrossRef]
- Tamirat, A.G.; Rick, J.; Dubale, A.A.; Su, W.N.; Hwang, B.J. Using hematite for photoelectrochemical water splitting: A review of current progress and challenges. Nanoscale Horizons 2016, 1, 243–267. [Google Scholar] [CrossRef] [PubMed]
- Leygraf, C.; Hendewerk, M.; Somorjai, G. The preparation and selected properties of Mg-doped p-type iron oxide as a photocathode for the photoelectrolysis of water using visible light. J. Solid State Chem. 1983, 48, 357–367. [Google Scholar] [CrossRef]
- Fan, H.M.; You, G.J.; Li, Y.; Zheng, Z.; Tan, H.R.; Shen, Z.X.; Tang, S.H.; Feng, Y.P. Shape-controlled synthesis of single-crystalline α-Fe2O3 hollow nanocrystals and their tunable optical properties. J. Phys. Chem. C 2009, 113, 9928–9935. [Google Scholar] [CrossRef]















| Sample | R1 | CPE1 | R2 |
|---|---|---|---|
| () | (×10 Fcm) | () | |
| pristine hematite | 57.9 | 7.7 | 1162.3 |
| -FeO:Zn | 53.2 | 7.5 | 808.5 |
| -FeO:Ag | 50.4 | 7.4 | 753.5 |
| -FeO:Zn/Ag | 50.3 | 4.2 | 749.8 |
| Sample | J (1.23V) | Donor Density | ||||
|---|---|---|---|---|---|---|
| (mAcm) | (fs) | (ps) | (ps) | (ns) | (×10 cm) | |
| Pristine hematite | 0.033 | 811 | 9 | 49 | 10 | 0.157 |
| -FeO:Zn | 0.160 | 797 | 9 | 97 | 4 | 5.63 |
| -FeO:Ag | 0.270 | 813 | 9 | 96 | 10 | 6.91 |
| -FeO:Zn/Ag | 0.470 | 1005 | 12 | 85 | 8 | 8.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nyarige, J.S.; Paradzah, A.T.; Krüger, T.P.J.; Diale, M. Mono-Doped and Co-Doped Nanostructured Hematite for Improved Photoelectrochemical Water Splitting. Nanomaterials 2022, 12, 366. https://doi.org/10.3390/nano12030366
Nyarige JS, Paradzah AT, Krüger TPJ, Diale M. Mono-Doped and Co-Doped Nanostructured Hematite for Improved Photoelectrochemical Water Splitting. Nanomaterials. 2022; 12(3):366. https://doi.org/10.3390/nano12030366
Chicago/Turabian StyleNyarige, Justine Sageka, Alexander T. Paradzah, Tjaart P. J. Krüger, and Mmantsae Diale. 2022. "Mono-Doped and Co-Doped Nanostructured Hematite for Improved Photoelectrochemical Water Splitting" Nanomaterials 12, no. 3: 366. https://doi.org/10.3390/nano12030366
APA StyleNyarige, J. S., Paradzah, A. T., Krüger, T. P. J., & Diale, M. (2022). Mono-Doped and Co-Doped Nanostructured Hematite for Improved Photoelectrochemical Water Splitting. Nanomaterials, 12(3), 366. https://doi.org/10.3390/nano12030366