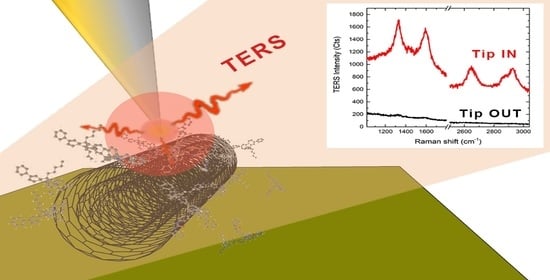
Comparing Commercial Metal-Coated AFM Tips and Home-Made Bulk Gold Tips for Tip-Enhanced Raman Spectroscopy of Polymer Functionalized Multiwalled Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polymer-Functionalized MWCNTs Synthesis
2.2. TERS Substrate Preparation
2.3. TERS Setup
3. Results and Discussion
3.1. Morphological Characterization of MWCNTs
3.2. TERS Enhancement of MWCNTs
3.3. TERS Resolution and Structural Characterization of MWCNTs
3.4. TERS Characterization of Polymer-Functionalized MWCNTs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novotny, L.; van Hulst, N. Antennas for light. Nat. Photonics 2011, 5, 83–90. [Google Scholar] [CrossRef]
- Novotny, L.; Hecht, B. Principles of Nano-Optics; Cambridge University Press: Cambridge, UK, 2012; ISBN 978-1-139-56045-0. [Google Scholar]
- Le Ru, E.C.; Etchegoin, P.G. Phenomenological local field enhancement factor distributions around electromagnetic hot spots. J. Chem. Phys. 2009, 130, 181101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, F.; Zenobi, R. Tip-enhanced Raman spectroscopy: Principles, practice, and applications to nanospectroscopic imaging of 2D materials. Anal. Bioanal. Chem. 2019, 411, 37–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deckert-Gaudig, T.; Taguchi, A.; Kawata, S.; Deckert, V. Tip-enhanced Raman spectroscopy—From early developments to recent advances. Chem. Soc. Rev. 2017, 46, 4077–4110. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, M.D.; Klingsporn, J.M.; Garibay, L.K.; Roberts, J.M.; Dieringer, J.A.; Seideman, T.; Scheidt, K.A.; Jensen, L.; Schatz, G.C.; van Duyne, R.P. Single-Molecule Tip-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2012, 116, 478–483. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, Y.; Zhang, R.; Hu, C.; Liao, M.; Luo, Y.; Yang, J.; Dong, Z.; Hou, J.G. Distinguishing adjacent molecules on a surface using plasmon-enhanced Raman scattering. Nat. Nanotechnol. 2015, 10, 865–869. [Google Scholar] [CrossRef]
- Wang, X.; Huang, S.-C.; Huang, T.-X.; Su, H.-S.; Zhong, J.-H.; Zeng, Z.-C.; Li, M.-H.; Ren, B. Tip-enhanced Raman spectroscopy for surfaces and interfaces. Chem. Soc. Rev. 2017, 46, 4020–4041. [Google Scholar] [CrossRef]
- Lee, J.; Crampton, K.T.; Tallarida, N.; Apkarian, V.A. Visualizing vibrational normal modes of a single molecule with atomically confined light. Nature 2019, 568, 78–82. [Google Scholar] [CrossRef]
- Richard-Lacroix, M.; Zhang, Y.; Dong, Z.; Deckert, V. Mastering high resolution tip-enhanced Raman spectroscopy: Towards a shift of perception. Chem. Soc. Rev. 2017, 46, 3922–3944. [Google Scholar] [CrossRef]
- Huang, T.-X.; Huang, S.-C.; Li, M.-H.; Zeng, Z.-C.; Wang, X.; Ren, B. Tip-enhanced Raman spectroscopy: Tip-related issues. Anal. Bioanal. Chem. 2015, 407, 8177–8195. [Google Scholar] [CrossRef]
- Rodriguez, R.D.; Sheremet, E.; Müller, S.; Gordan, O.D.; Villabona, A.; Schulze, S.; Hietschold, M.; Zahn, D.R.T. Compact metal probes: A solution for atomic force microscopy based tip-enhanced Raman spectroscopy. Rev. Sci. Instrum. 2012, 83, 123708. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.D.; Lopez, R.; Meyer, H.; Feldman, L.C.; Haglund, R.F. Rapid tarnishing of silver nanoparticles in ambient laboratory air. Appl. Phys. A 2005, 80, 915–921. [Google Scholar] [CrossRef]
- Opilik, L.; Dogan, Ü.; Li, C.-Y.; Stephanidis, B.; Li, J.-F.; Zenobi, R. Chemical Production of Thin Protective Coatings on Optical Nanotips for Tip-Enhanced Raman Spectroscopy. J. Phys. Chem. C 2016, 120, 20828–20832. [Google Scholar] [CrossRef]
- Yang, L.-K.; Huang, T.-X.; Zeng, Z.-C.; Li, M.-H.; Wang, X.; Yang, F.-Z.; Ren, B. Rational fabrication of a gold-coated AFM TERS tip by pulsed electrodeposition. Nanoscale 2015, 7, 18225–18231. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-X.; Li, C.-W.; Yang, L.-K.; Zhu, J.-F.; Yao, X.; Liu, C.; Lin, K.-Q.; Zeng, Z.-C.; Wu, S.-S.; Wang, X.; et al. Rational fabrication of silver-coated AFM TERS tips with a high enhancement and long lifetime. Nanoscale 2018, 10, 4398–4405. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, A.; Yu, J.; Verma, P.; Kawata, S. Optical antennas with multiple plasmonic nanoparticles for tip-enhanced Raman microscopy. Nanoscale 2015, 7, 17424–17433. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Calogero, G.; Di Marco, G.; Maragò, O.M.; Gucciardi, P.G.; Giorgianni, U.; Channon, K.; Sabatino, G. Fabrication of gold tips by chemical etching in aqua regia. Rev. Sci. Instrum. 2007, 78, 103702. [Google Scholar] [CrossRef]
- Ren, B.; Picardi, G.; Pettinger, B. Preparation of gold tips suitable for tip-enhanced Raman spectroscopy and light emission by electrochemical etching. Rev. Sci. Instrum. 2004, 75, 837–841. [Google Scholar] [CrossRef]
- Lopes, M.; Toury, T.; de La Chapelle, M.L.; Bonaccorso, F.; Gucciardi, P.G. Fast and reliable fabrication of gold tips with sub-50 nm radius of curvature for tip-enhanced Raman spectroscopy. Rev. Sci. Instrum. 2013, 84, 073702. [Google Scholar] [CrossRef]
- Foti, A.; Barreca, F.; Fazio, E.; D’Andrea, C.; Matteini, P.; Maragò, O.M.; Gucciardi, P.G. Low cost tips for tip-enhanced Raman spectroscopy fabricated by two-step electrochemical etching of 125 µm diameter gold wires. Beilstein J. Nanotechnol. 2018, 9, 2718–2729. [Google Scholar] [CrossRef] [Green Version]
- Borromeo, L.; Toccafondi, C.; Minde, M.W.; Zimmermann, U.; Andò, S.; Madland, M.V.; Korsnes, R.I.; Ossikovski, R. Application of Tip-Enhanced Raman Spectroscopy for the nanoscale characterization of flooded chalk. J. Appl. Phys. 2018, 124, 173101. [Google Scholar] [CrossRef]
- Picardi, G.; Nguyen, Q.; Schreiber, J.; Ossikovski, R. Comparative study of atomic force mode and tunneling mode tip-enhanced Raman spectroscopy. Eur. Phys. J. Appl. Phys. 2007, 40, 197–201. [Google Scholar] [CrossRef]
- Gao, L.; Zhao, H.; Li, Y.; Li, T.; Chen, D.; Liu, B. Controllable Fabrication of Au-Coated AFM Probes via a Wet-Chemistry Procedure. Nanoscale Res. Lett. 2018, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- IRIS TERS Probes—BRUKER. Available online: https://www.brukerafmprobes.com/t-IRIS-TERS-Probes.aspx (accessed on 27 November 2021).
- TERS Probes—HORIBA. Available online: https://www.horiba.com/en_en/products/detail/action/show/Product/ters-probes-1634/ (accessed on 27 November 2021).
- TERS AFM Probes—NT-MDT Tips. Available online: https://www.ntmdt-tips.com/products/group/ters-afm-probes-new (accessed on 27 November 2021).
- Sharma, G.; Deckert-Gaudig, T.; Deckert, V. Tip-enhanced Raman scattering—Targeting structure-specific surface characterization for biomedical samples. Adv. Drug Deliv. Rev. 2015, 89, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Bonhommeau, S.; Talaga, D.; Hunel, J.; Cullin, C.; LeComte, S. Tip-Enhanced Raman Spectroscopy to Distinguish Toxic Oligomers from Aβ1-42 Fibrils at the Nanometer Scale. Angew. Chem. 2017, 129, 1797–1800. [Google Scholar] [CrossRef]
- D’Andrea, C.; Foti, A.; Cottat, M.; Banchelli, M.; Capitini, C.; Barreca, F.; Canale, C.; de Angelis, M.; Relini, A.; Marago, O.M.; et al. Nanoscale Discrimination between Toxic and Nontoxic Protein Misfolded Oligomers with Tip-Enhanced Raman Spectroscopy. Small 2018, 14, e1800890. [Google Scholar] [CrossRef]
- Lucas, M.; Riedo, E. Invited Review Article: Combining scanning probe microscopy with optical spectroscopy for applications in biology and materials science. Rev. Sci. Instrum. 2012, 83, 061101. [Google Scholar] [CrossRef] [Green Version]
- Wood, B.R.; Asghari-Khiavi, M.; Bailo, E.; McNaughton, D.; Deckert, V. Detection of Nano-Oxidation Sites on the Surface of Hemoglobin Crystals Using Tip-Enhanced Raman Scattering. Nano Lett. 2012, 12, 1555–1560. [Google Scholar] [CrossRef]
- Domke, K.F.; Zhang, D.; Pettinger, B. Tip-Enhanced Raman Spectra of Picomole Quantities of DNA Nucleobases at Au(111). J. Am. Chem. Soc. 2007, 129, 6708–6709. [Google Scholar] [CrossRef]
- Paulite, M.; Blum, C.; Schmid, T.; Opilik, L.; Eyer, K.; Walker, G.; Zenobi, R. Full Spectroscopic Tip-Enhanced Raman Imaging of Single Nanotapes Formed from β-Amyloid(1–40) Peptide Fragments. ACS Nano 2013, 7, 911–920. [Google Scholar] [CrossRef]
- Naumenko, D.; Snitka, V.; Serviene, E.; Bruzaite, I.; Snopok, B. In vivo characterization of protein uptake by yeast cell envelope: Single cell AFM imaging and μ-tip-enhanced Raman scattering study. Analyst 2013, 138, 5371–5383. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.-C.; Huang, S.-C.; Wu, D.-Y.; Meng, L.-Y.; Li, M.-H.; Huang, T.-X.; Zhong, J.-H.; Wang, X.; Yang, Z.-L.; Ren, B. Electrochemical Tip-Enhanced Raman Spectroscopy. J. Am. Chem. Soc. 2015, 137, 11928–11931. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Lan, J.-G.; Goubert, G.; Wang, Y.-H.; Li, J.-F.; Zenobi, R. Nanoscale Surface Redox Chemistry Triggered by Plasmon-Generated Hot Carriers. Small 2019, 15, 1903674. [Google Scholar] [CrossRef] [PubMed]
- Sartin, M.M.; Su, H.-S.; Wang, X.; Ren, B. Tip-enhanced Raman spectroscopy for nanoscale probing of dynamic chemical systems. J. Chem. Phys. 2020, 153, 170901. [Google Scholar] [CrossRef] [PubMed]
- Berweger, S.; Neacsu, C.C.; Mao, Y.; Zhou, H.; Wong, S.S.; Raschke, M.B. Optical nanocrystallography with tip-enhanced phonon Raman spectroscopy. Nat. Nanotechnol. 2009, 4, 496–499. [Google Scholar] [CrossRef]
- Böhmler, M.; Wang, Z.; Myalitsin, A.; Mews, A.; Hartschuh, A. Optical Imaging of CdSe Nanowires with Nanoscale Resolution. Angew. Chem. Int. Ed. 2011, 50, 11536–11538. [Google Scholar] [CrossRef]
- Picardi, G.; Chaigneau, M.; Ossikovski, R.; Licitra, C.; Delapierre, G. Tip enhanced Raman spectroscopy on azobenzene thiol self-assembled monolayers on Au(111). J. Raman Spectrosc. 2009, 40, 1407–1412. [Google Scholar] [CrossRef]
- Picardi, G.; Królikowska, A.; Yasukuni, R.; Chaigneau, M.; Escude, M.; Mourier, V.; Licitra, C.; Ossikovski, R. Exchange of Methyl- and Azobenzene-Terminated Alkanethiols on Polycrystalline Gold Studied by Tip-Enhanced Raman Mapping. ChemPhysChem 2014, 15, 276–282. [Google Scholar] [CrossRef]
- Wickramasinghe, H.K.; Chaigneau, M.; Yasukuni, R.; Picardi, G.; Ossikovski, R. Billion-Fold Increase in Tip-Enhanced Raman Signal. ACS Nano 2014, 8, 3421–3426. [Google Scholar] [CrossRef]
- Toccafondi, C.; Picardi, G.; Ossikovski, R. Molecular Bending at the Nanoscale Evidenced by Tip-Enhanced Raman Spectroscopy in Tunneling Mode on Thiol Self-Assembled Monolayers. J. Phys. Chem. C 2016, 120, 18209–18219. [Google Scholar] [CrossRef]
- Foti, A.; Toccafondi, C.; Ossikovski, R. Study of the Molecular Bending in Azobenzene Self-Assembled Monolayers Observed by Tip-Enhanced Raman Spectroscopy in Scanning Tunneling Mode. J. Phys. Chem. C 2019, 123, 26554–26563. [Google Scholar] [CrossRef]
- Chen, C.; Hayazawa, N.; Kawata, S. A 1.7 nm resolution chemical analysis of carbon nanotubes by tip-enhanced Raman imaging in the ambient. Nat. Commun. 2014, 5, 3312. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.-A.; Ichimura, T.; Kuwahara, S.; H’Dhili, F.; Uetsuki, K.; Okuno, Y.; Verma, P.; Kawata, S. Tip-enhanced nano-Raman analytical imaging of locally induced strain distribution in carbon nanotubes. Nat. Commun. 2013, 4, 2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, M.; Jiang, S.; Hu, C.-R.; Zhang, R.; Kuang, Y.-M.; Zhu, J.-Z.; Zhang, Y.; Dong, Z. Tip-Enhanced Raman Spectroscopic Imaging of Individual Carbon Nanotubes with Subnanometer Resolution. Nano Lett. 2016, 16, 4040–4046. [Google Scholar] [CrossRef]
- Chaunchaiyakul, S.; Yano, T.; Khoklang, K.; Krukowski, P.; Akai-Kasaya, M.; Saito, A.; Kuwahara, Y. Nanoscale analysis of multiwalled carbon nanotube by tip-enhanced Raman spectroscopy. Carbon 2016, 99, 642–648. [Google Scholar] [CrossRef]
- Picardi, G.; Chaigneau, M.; Ossikovski, R. High resolution probing of multi wall carbon nanotubes by Tip Enhanced Raman Spectroscopy in gap-mode. Chem. Phys. Lett. 2008, 469, 161–165. [Google Scholar] [CrossRef]
- Stadler, J.; Schmid, T.; Zenobi, R. Nanoscale Chemical Imaging of Single-Layer Graphene. ACS Nano 2011, 5, 8442–8448. [Google Scholar] [CrossRef]
- Beams, R.; Cançado, L.G.; Jorio, A.; Vamivakas, A.N.; Novotny, L. Tip-enhanced Raman mapping of local strain in graphene. Nanotechnology 2015, 26, 175702. [Google Scholar] [CrossRef] [Green Version]
- Bhattarai, A.; Krayev, A.; Temiryazev, A.; Evplov, D.; Crampton, K.T.; Hess, W.P.; El-Khoury, P.Z. Tip-Enhanced Raman Scattering from Nanopatterned Graphene and Graphene Oxide. Nano Lett. 2018, 18, 4029–4033. [Google Scholar] [CrossRef]
- Kurouski, D.; Zaleski, S.; Casadio, F.; Van Duyne, R.P.; Shah, N.C. Tip-Enhanced Raman Spectroscopy (TERS) for in Situ Identification of Indigo and Iron Gall Ink on Paper. J. Am. Chem. Soc. 2014, 136, 8677–8684. [Google Scholar] [CrossRef]
- Su, W.; Kumar, N.; Krayev, A.; Chaigneau, M. In situ topographical chemical and electrical imaging of carboxyl graphene oxide at the nanoscale. Nat. Commun. 2018, 9, 2891. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Pitner, G.; Giri, G.; Koo, J.H.; Park, J.; Kim, K.; Wang, H.; Sinclair, R.; Wong, H.-S.P.; Bao, Z. Large-Area Assembly of Densely Aligned Single-Walled Carbon Nanotubes Using Solution Shearing and Their Application to Field-Effect Transistors. Adv. Mater. 2015, 27, 2656–2662. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, J.L. Semiconducting Single-Walled Carbon Nanotubes in Solar Energy Harvesting. ACS Energy Lett. 2017, 2, 1598–1613. [Google Scholar] [CrossRef]
- Ravindran, S.; Chaudhary, S.; Colburn, B.; Ozkan, M.; Ozkan, C.S. Covalent Coupling of Quantum Dots to Multiwalled Carbon Nanotubes for Electronic Device Applications. Nano Lett. 2003, 3, 447–453. [Google Scholar] [CrossRef]
- Ago, H.; Petritsch, K.; Shaffer, M.S.P.; Windle, A.H.; Friend, R.H. Composites of Carbon Nanotubes and Conjugated Polymers for Photovoltaic Devices. Adv. Mater. 1999, 11, 1281–1285. [Google Scholar] [CrossRef]
- Frogley, M.D.; Ravich, D.; Wagner, H.D. Mechanical properties of carbon nanoparticle-reinforced elastomers. Compos. Sci. Technol. 2003, 63, 1647–1654. [Google Scholar] [CrossRef]
- Bokobza, L. Mechanical, electrical and spectroscopic investigations of carbon nanotube-reinforced elastomers. Vib. Spectrosc. 2009, 51, 52–59. [Google Scholar] [CrossRef]
- Messina, E.; Leone, N.; Foti, A.; Di Marco, G.; Riccucci, C.; Di Carlo, G.; Di Maggio, F.; Cassata, A.; Gargano, L.; D’Andrea, C.; et al. Double-Wall Nanotubes and Graphene Nanoplatelets for Hybrid Conductive Adhesives with Enhanced Thermal and Electrical Conductivity. ACS Appl. Mater. Interfaces 2016, 8, 23244–23259. [Google Scholar] [CrossRef]
- Coleman, J.N.; Khan, U.; Blau, W.J.; Gun’Ko, Y.K. Small but strong: A review of the mechanical properties of carbon nanotube–polymer composites. Carbon 2006, 44, 1624–1652. [Google Scholar] [CrossRef]
- Zhou, C.; Qiu, X.; Zhuang, Q.; Han, Z.; Wu, Q. In situ polymerization and photophysical properties of poly(p-phenylene benzobisoxazole)/multiwalled carbon nanotubes composites. J. Appl. Polym. Sci. 2011, 124, 4740–4746. [Google Scholar] [CrossRef]
- Fong, D.; Yeung, J.; Meichsner, E.; Adronov, A. Reactive, Aqueous-Dispersible Polyfluorene-Wrapped Carbon Nanotubes Modulated with an Acidochromic Switch via Azide–Alkyne Cycloaddition. ACS Appl. Polym. Mater. 2019, 1, 797–803. [Google Scholar] [CrossRef]
- Eguílaz, M.; Gutiérrez, A.; Rivas, G. Non-covalent functionalization of multi-walled carbon nanotubes with cytochrome c: Enhanced direct electron transfer and analytical applications. Sens. Actuators B Chem. 2016, 225, 74–80. [Google Scholar] [CrossRef]
- Verma, S.K.; Kar, P.; Yang, D.J.; Choudhury, A. Poly(m-aminophenol)/functionalized multi-walled carbon nanotube nanocomposite based alcohol sensors. Sens. Actuators B Chem. 2015, 219, 199–208. [Google Scholar] [CrossRef]
- Salazar-Rios, J.M.; Talsma, W.; Derenskyi, V.; Gomulya, W.; Keller, T.; Fritsch, M.; Kowalski, S.; Preis, E.; Wang, M.; Allard, S.; et al. Understanding the Selection Mechanism of the Polymer Wrapping Technique toward Semiconducting Carbon Nanotubes. Small Methods 2018, 2, 1700335. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Yan, X.; Kitahama, Y.; Sato, H.; Itoh, T.; Miura, T.; Ozaki, Y. Tip-Enhanced Raman Spectroscopy Study of Local Interactions at the Interface of Styrene–Butadiene Rubber/Multiwalled Carbon Nanotube Nanocomposites. J. Phys. Chem. C 2013, 117, 1436–1440. [Google Scholar] [CrossRef]
- Benda, R.; Zucchi, G.; Cancès, E.; Lebental, B. Insights into the π–π interaction driven non-covalent functionalization of carbon nanotubes of various diameters by conjugated fluorene and carbazole copolymers. J. Chem. Phys. 2020, 152, 064708. [Google Scholar] [CrossRef]
- Yan, X.; Suzuki, T.; Kitahama, Y.; Sato, H.; Itoh, T.; Ozaki, Y. A study on the interaction of single-walled carbon nanotubes (SWCNTs) and polystyrene (PS) at the interface in SWCNT–PS nanocomposites using tip-enhanced Raman spectroscopy. Phys. Chem. Chem. Phys. 2013, 15, 20618–20624. [Google Scholar] [CrossRef]
- Michelis, F.; Bodelot, L.; Bonnassieux, Y.; Lebental, B. Highly reproducible, hysteresis-free, flexible strain sensors by inkjet printing of carbon nanotubes. Carbon 2015, 95, 1020–1026. [Google Scholar] [CrossRef] [Green Version]
- Zucchi, G.; Lebental, B.; Loisel, L.; Ramachandran, S.; Gutierrez, A.F.; Wang, X.; Godumala, M.; Bodelot, L. Chemical Sensors Based on Carbon Nanotubes Functionalised by Conjugated Polymers for Analysis in Aqueous Medium. World Patent Application WO2018189479A1, 18 October 2018. [Google Scholar]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Jorio, A.; Saito, R. Raman spectroscopy for carbon nanotube applications. J. Appl. Phys. 2021, 129, 021102. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, R.; Igarashi, S.; Umakoshi, T.; Verma, P. Tip-Enhanced Raman Spectroscopy of Multiwalled Carbon Nanotubes through D-Band Imaging: Implications for Nanoscale Analysis of Interwall Interactions. ACS Appl. Nano Mater. 2020, 3, 6001–6008. [Google Scholar] [CrossRef]
- Meng, L.; Huang, T.; Wang, X.; Chen, S.; Yang, Z.; Ren, B. Gold-coated AFM tips for tip-enhanced Raman spectroscopy: Theoretical calculation and experimental demonstration. Opt. Express 2015, 23, 13804–13813. [Google Scholar] [CrossRef] [PubMed]
- Jaculbia, R.B.; Imada, H.; Miwa, K.; Iwasa, T.; Takenaka, M.; Yang, B.; Kazuma, E.; Hayazawa, N.; Taketsugu, T.; Kim, Y. Single-molecule resonance Raman effect in a plasmonic nanocavity. Nat. Nanotechnol. 2020, 15, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Benz, F.; Schmidt, M.K.; Dreismann, A.; Chikkaraddy, R.; Zhang, Y.; Demetriadou, A.; Carnegie, C.; Ohadi, H.; de Nijs, B.; Esteban, R.; et al. Single-molecule optomechanics in “picocavities”. Science 2016, 354, 726–729. [Google Scholar] [CrossRef] [Green Version]
- Chaigneau, M.; Picardi, G.; Ossikovski, R. Tip enhanced Raman spectroscopy evidence for amorphous carbon contamination on gold surfaces. Surf. Sci. 2010, 604, 701–705. [Google Scholar] [CrossRef]
- Dileo, R.A.; Landi, B.J.; Raffaelle, R.P. Purity assessment of multiwalled carbon nanotubes by Raman spectroscopy. J. Appl. Phys. 2007, 101, 064307. [Google Scholar] [CrossRef] [Green Version]
- Cronin, S.B.; Swan, A.K.; Ünlü, M.S.; Goldberg, B.B.; Dresselhaus, M.S.; Tinkham, M. Measuring the Uniaxial Strain of Individual Single-Wall Carbon Nanotubes: Resonance Raman Spectra of Atomic-Force-Microscope Modified Single-Wall Nanotubes. Phys. Rev. Lett. 2004, 93, 167401. [Google Scholar] [CrossRef] [Green Version]
- Cooper, C.; Young, R.; Halsall, M. Investigation into the deformation of carbon nanotubes and their composites through the use of Raman spectroscopy. Compos. Part A Appl. Sci. Manuf. 2001, 32, 401–411. [Google Scholar] [CrossRef]
- Zeng, C.; Hossieny, N.; Zhang, C.; Wang, B.; Walsh, S.M. Morphology and tensile properties of PMMA carbon nanotubes nanocomposites and nanocomposites foams. Compos. Sci. Technol. 2013, 82, 29–37. [Google Scholar] [CrossRef]
- Breuer, O.; Sundararaj, U. Big returns from small fibers: A review of polymer/carbon nanotube composites. Polym. Compos. 2004, 25, 630–645. [Google Scholar] [CrossRef]
- Ariu, M.; Lidzey, D.G.; Lavrentiev, M.; Bradley, D.D.C.; Jandke, M.; Strohriegl, P. A study of the different structural phases of the polymer poly(9,9′-dioctyl fluorene) using Raman spectroscopy. Synth. Met. 2001, 116, 217–221. [Google Scholar] [CrossRef]
- Witt, K. Vibrational analysis of fluorene. Spectrochim. Acta Part A Mol. Spectrosc. 1968, 24, 1115–1123. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, S.; Hamon, M.A.; Perea, D.E.; Kang, C.B.; Zhao, B.; Pal, S.K.; Wyant, A.E.; Itkis, A.M.E.; Haddon, R.C. Ultrasonic Dispersions of Single-Walled Carbon Nanotubes. J. Phys. Chem. B 2003, 107, 8799–8804. [Google Scholar] [CrossRef]
- Bokobza, L. Vibrational Spectroscopic and Mechanical Investigation of Carbon Nanotube-Reinforced Styrene-Butadiene Rubbers. Macromol. Symp. 2011, 305, 1–9. [Google Scholar] [CrossRef]
- Yan, X.; Kitahama, Y.; Sato, H.; Suzuki, T.; Han, X.; Itoh, T.; Bokobza, L.; Ozaki, Y. Laser heating effect on Raman spectra of styrene–butadiene rubber/multiwalled carbon nanotube nanocomposites. Chem. Phys. Lett. 2012, 523, 87–91. [Google Scholar] [CrossRef]
- Wood, J.; Frogley, M.D.; Meurs, E.R.; Prins, A.D.; Peijs, T.; Dunstan, D.J.; Wagner, H.D. Mechanical Response of Carbon Nanotubes under Molecular and Macroscopic Pressures. J. Phys. Chem. B 1999, 103, 10388–10392. [Google Scholar] [CrossRef]
- Yashiro, S.; Sakaida, Y.; Shimamura, Y.; Inoue, Y. Evaluation of interfacial shear stress between multi-walled carbon nanotubes and epoxy based on strain distribution measurement using Raman spectroscopy. Compos. Part A Appl. Sci. Manuf. 2016, 85, 192–198. [Google Scholar] [CrossRef] [Green Version]
- Lucas, M.; Young, R. Raman spectroscopic study of the effect of strain on the radial breathing modes of carbon nanotubes in epoxy/SWNT composites. Compos. Sci. Technol. 2004, 64, 2297–2302. [Google Scholar] [CrossRef]
- Liu, J.; Li, Q.; Zou, Y.; Qian, Q.; Jin, Y.; Li, G.; Jiang, K.; Fan, S. The Dependence of Graphene Raman D-band on Carrier Density. Nano Lett. 2013, 13, 6170–6175. [Google Scholar] [CrossRef] [PubMed]
- Pócsik, I.; Hundhausen, M.; Koós, M.; Ley, L. Origin of the D peak in the Raman spectrum of microcrystalline graphite. J. Non-Cryst. Solids 1998, 227–230, 1083–1086. [Google Scholar] [CrossRef]
- Dresselhaus, M.; Dresselhaus, G.; Jorio, A.; Filho, A.S.; Saito, R. Raman spectroscopy on isolated single wall carbon nanotubes. Carbon 2002, 40, 2043–2061. [Google Scholar] [CrossRef]
- Chen, Y.; Tao, J.; Li, S.; Khashab, N.M. Compositing Polyetherimide with Polyfluorene Wrapped Carbon Nanotubes for Enhanced Interfacial Interaction and Conductivity. ACS Appl. Mater. Interfaces 2014, 6, 9013–9022. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foti, A.; Venkatesan, S.; Lebental, B.; Zucchi, G.; Ossikovski, R. Comparing Commercial Metal-Coated AFM Tips and Home-Made Bulk Gold Tips for Tip-Enhanced Raman Spectroscopy of Polymer Functionalized Multiwalled Carbon Nanotubes. Nanomaterials 2022, 12, 451. https://doi.org/10.3390/nano12030451
Foti A, Venkatesan S, Lebental B, Zucchi G, Ossikovski R. Comparing Commercial Metal-Coated AFM Tips and Home-Made Bulk Gold Tips for Tip-Enhanced Raman Spectroscopy of Polymer Functionalized Multiwalled Carbon Nanotubes. Nanomaterials. 2022; 12(3):451. https://doi.org/10.3390/nano12030451
Chicago/Turabian StyleFoti, Antonino, Suriya Venkatesan, Bérengère Lebental, Gaël Zucchi, and Razvigor Ossikovski. 2022. "Comparing Commercial Metal-Coated AFM Tips and Home-Made Bulk Gold Tips for Tip-Enhanced Raman Spectroscopy of Polymer Functionalized Multiwalled Carbon Nanotubes" Nanomaterials 12, no. 3: 451. https://doi.org/10.3390/nano12030451
APA StyleFoti, A., Venkatesan, S., Lebental, B., Zucchi, G., & Ossikovski, R. (2022). Comparing Commercial Metal-Coated AFM Tips and Home-Made Bulk Gold Tips for Tip-Enhanced Raman Spectroscopy of Polymer Functionalized Multiwalled Carbon Nanotubes. Nanomaterials, 12(3), 451. https://doi.org/10.3390/nano12030451