Validation and Demonstration of an Atmosphere-Temperature-pH-Controlled Stirred Batch Reactor System for Determination of (Nano)Material Solubility and Dissolution Kinetics in Physiological Simulant Lung Fluids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanomaterials
2.2. Wavelength Dispersive X-ray Fluorescence Spectroscopy
2.3. Thermogravimetric Analysis
2.4. X-ray Diffraction
2.5. Physiological Relevant Fluids
2.6. Dispersion of Nanomaterials
2.7. Dynamic Light Scattering and Laser Doppler Electrophoresis
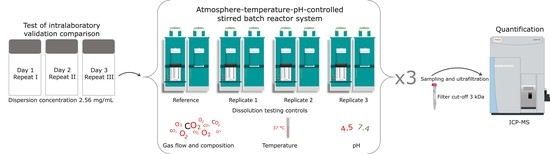
2.8. Atmosphere-Temperature-pH-Controlled Stirred Batch Reactor System
2.9. Inductively Coupled Plasma-Mass Spectrometry
2.10. Determination of Initial Dissolution Rates
2.11. Reactivity
2.12. Statistics
3. Results and Discussion
3.1. Physicochemical Characteristics of the Test Materials
3.2. Repeatability and Robustness
3.3. Particle Dispersion
3.4. Reactivity
3.5. Repeatability of the ATempH SBR System
3.6. Dissolution in Phagolysosomal Fluid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heiligtag, F.J.; Niederberger, M. The fascinating world of nanoparticle research. Mater. Today 2013, 16, 262–271. [Google Scholar] [CrossRef]
- Kuhlbusch, T.A.J.; Asbach, C.; Fissan, H.; Göhler, D.; Stintz, M. Nanoparticle exposure at nanotechnology workplaces: A review. Part. Fibre Toxicol. 2011, 8, 262–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Kumar, K.S.; Choudhary, N.; Jung, Y.; Thomas, J. Recent Advances in Two-Dimensional Nanomaterials for Supercapacitor Electrode Applications. ACS Energy Lett. 2018, 3, 482–495. [Google Scholar] [CrossRef]
- Sozer, N.; Kokini, J.L. Nanotechnology and its applications in the food sector. Trends Biotechnol. 2009, 27, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Gruère, G.P. Implications of nanotechnology growth in food and agriculture in OECD countries. Food Policy 2012, 37, 191–198. [Google Scholar] [CrossRef]
- Roco, M.C. International strategy for nanotechnology research and development. J. Nanopart. Res. 2001, 3, 353–360. [Google Scholar] [CrossRef]
- Official Journal of the European Union. Commission Recommendation of 18 October 2011 on the Definition of Nanomaterial Text with EEA Relevance OJ L 275. 2011. Available online: https://op.europa.eu/en/publication-detail/-/publication/17af73d9-da70-4a46-a421-c62e3d1df6ce/language-en (accessed on 12 December 2021).
- Official Journal of the European Union. Commission Regulation (EU) 2018/1881 of 3 December 2018 Amending Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Annexes I, III, VI, V. 2018. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32018R1881 (accessed on 12 December 2021).
- Thit, A.; Skjolding, L.M.; Selck, H.; Sturve, J. Effects of copper oxide nanoparticles and copper ions to zebrafish (Danio rerio) cells, embryos and fry. Toxicol. Vitr. 2017, 45, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 2018, 30, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Nowack, B.; Bucheli, T.D. Occurrence, behavior and effects of nanoparticles in the environment. Environ. Pollut. 2007, 150, 5–22. [Google Scholar] [CrossRef]
- Wigger, H.; Wohlleben, W.; Nowack, B. Redefining environmental nanomaterial flows: Consequences of the regulatory nanomaterial definition on the results of environmental exposure models. Environ. Sci. Nano 2018, 5, 1372–1385. [Google Scholar] [CrossRef]
- Anand, O.; Yu, L.X.; Conner, D.P.; Davit, B.M. Dissolution testing for generic drugs: An FDA perspective. AAPS J. 2011, 13, 328–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azarmi, S.; Roa, W.; Löbenberg, R. Current perspectives in dissolution testing of conventional and novel dosage forms. Int. J. Pharm. 2007, 328, 12–21. [Google Scholar] [CrossRef]
- Tedeschi, C.; Clement, V.; Rouvet, M.; Valles-Pamies, B. Dissolution tests as a tool for predicting bioaccessibility of nutrients during digestion. Food Hydrocoll. 2009, 23, 1228–1235. [Google Scholar] [CrossRef]
- Scientific Committee on Consumer Safety. Guidance on the Safety Assessment of Nanomaterials in Cosmetics. SCCS/1611/19. 2019. Available online: https://ec.europa.eu/health/system/files/2020-10/sccs_o_233_0.pdf (accessed on 12 December 2021).
- Stone, V.; Gottardo, S.; Bleeker, E.A.J.; Braakhuis, H.; Dekkers, S.; Fernandes, T.; Haase, A.; Hunt, N.; Hristozov, D.; Jantunen, P.; et al. A framework for grouping and read-across of nanomaterials-supporting innovation and risk assessment. Nano Today 2020, 35, 100941. [Google Scholar] [CrossRef]
- Braakhuis, H.M.; Murphy, F.; Ma-Hock, L.; Dekkers, S.; Keller, J.; Oomen, A.G.; Stone, V. An Integrated Approach to Testing and Assessment to Support Grouping and Read-Across of Nanomaterials after Inhalation Exposure. Appl. Vitr. Toxicol. 2021, 7, 112–128. [Google Scholar] [CrossRef]
- Tantra, R.; Bouwmeester, H.; Bolea, E.; Rey-Castro, C.; David, C.A.; Dogné, J.-M.; Jarman, J.; Laborda, F.; Laloy, J.; Robinson, K.N.; et al. Suitability of analytical methods to measure solubility for the purpose of nanoregulation. Nanotoxicology 2016, 10, 173–184. [Google Scholar] [CrossRef]
- Misra, S.K.; Dybowska, A.; Berhanu, D.; Luoma, S.N.; Valsami-Jones, E. The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. Sci. Total Environ. 2012, 438, 225–232. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA) Appendix for nanoforms applicable to the Guidance on Registration and substance identification. 2019. Available online: https://echa.europa.eu/documents/10162/13655/how_to_register_nano_en.pdf/f8c046ec-f60b-4349-492b-e915fd9e3ca0 (accessed on 12 December 2021).
- Rasmussen, K.; Rauscher, H.; Kearns, P.; González, M.; Riego Sintes, J. Developing OECD test guidelines for regulatory testing of nanomaterials to ensure mutual acceptance of test data. Regul. Toxicol. Pharmacol. 2019, 104, 74–83. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines and HealthCare (EDQM). 5.17.1 Recommendation on dissolution testing. European Pharmacopoeia Online 8.2. In Proceedings of the Training Session “The European Pharmacopoeia”, Iselin, NJ, USA, 10–11 September 2019. [Google Scholar]
- The United States Pharmacopoeia and National Formulary USP 37-NF 32. <711> Dissolution. 2011. Available online: https://www.usp.org/sites/default/files/usp/document/harmonization/gen-method/stage_6_monograph_25_feb_2011.pdf (accessed on 12 December 2021).
- Wohlleben, W.; Waindok, H.; Daumann, B.; Werle, K.; Drum, M.; Egenolf, H. Composition, Respirable Fraction and Dissolution Rate of 24 Stone Wool MMVF with their Binder. Part. Fibre Toxicol. 2017, 14, 29. [Google Scholar] [CrossRef] [Green Version]
- Guldberg, M.; Madsen, A.L.; Sebastian, K.; Fellmann, J.; Potter, R.; Bauer, J.; Searl, A.; Maquin, B.; Jubb, G. In-vitro dissolution of vitreous silicate fibres according to EURIMA test guideline—Results of two Round Robins. Glas. Sci. Technol. 2003, 76, 199–205. [Google Scholar]
- Christensen, V.R.; Lund Jensen, S.; Guldberg, M.; Kamstrup, O. Effect of chemical composition of man-made vitreous fibers on the rate of dissolution in vitro at different pHs. Environ. Health Perspect. 1994, 102, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Guldberg, M.; Christensen, V.R.; Krøis, W.; Sebastian, C. Method for determining in-vitro dissolution rates of man-made vitreous fibres. Glas. Sci. Technol. 1995, 68, 181. [Google Scholar]
- More, S.; Bampidis, V.; Benford, D.; Bragard, C.; Halldorsson, T.; Hernández-Jerez, A.; Bennekou, S.H.; Koutsoumanis, K.; Lambré, C.; Machera, K.; et al. Guidance on technical requirements for regulated food and feed product applications to establish the presence of small particles including nanoparticles. EFSA J. 2021, 19, 6769. [Google Scholar] [CrossRef]
- Koltermann-Jülly, J.; Keller, J.G.; Vennemann, A.; Werle, K.; Müller, P.; Ma-Hock, L.; Landsiedel, R.; Wiemann, M.; Wohlleben, W. Abiotic dissolution rates of 24 (nano)forms of 6 substances compared to macrophage-assisted dissolution and in vivo pulmonary clearance: Grouping by biodissolution and transformation. NanoImpact 2018, 12, 29–41. [Google Scholar] [CrossRef]
- Cho, W.S.; Duffin, R.; Thielbeer, F.; Bradley, M.; Megson, I.L.; MacNee, W.; Poland, C.A.; Tran, C.L.; Donaldson, K. Zeta potential and solubility to toxic ions as mechanisms of lung inflammation caused by metal/metal oxide nanoparticles. Toxicol. Sci. 2012, 126, 469–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberdörster, G.; Kuhlbusch, T.A.J. In vivo effects: Methodologies and biokinetics of inhaled nanomaterials. NanoImpact 2018, 10, 38–60. [Google Scholar] [CrossRef]
- Arts, J.H.E.; Hadi, M.; Irfan, M.A.; Keene, A.M.; Kreiling, R.; Lyon, D.; Maier, M.; Michel, K.; Petry, T.; Sauer, U.G.; et al. A decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping). Regul. Toxicol. Pharmacol. 2015, 71, S1–S27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avramescu, M.L.; Rasmussen, P.E.; Chénier, M.; Gardner, H.D. Influence of pH, particle size and crystal form on dissolution behaviour of engineered nanomaterials. Environ. Sci. Pollut. Res. 2017, 24, 1553–1564. [Google Scholar] [CrossRef] [Green Version]
- Borm, P.; Klaessig, F.C.; Landry, T.D.; Moudgil, B.; Pauluhn, J.; Thomas, K.; Trottier, R.; Wood, S. Research strategies for safety evaluation of nanomaterials, part V: Role of dissolution in biological fate and effects of nanoscale particles. Toxicol. Sci. 2006, 90, 23–32. [Google Scholar] [CrossRef] [Green Version]
- Bierkandt, F.S.; Leibrock, L.; Wagener, S.; Laux, P.; Luch, A. The impact of nanomaterial characteristics on inhalation toxicity. Toxicol. Res. 2018, 7, 321–346. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Kuhlbusch, T.A.J.; Van Tongeren, M.; Jiménez, A.S.; Tuinman, I.; Chen, R.; Alvarez, I.L.; Mikolajczyk, U.; Nickel, C.; Meyer, J.; et al. Airborne engineered nanomaterials in the workplace—A review of release and worker exposure during nanomaterial production and handling processes. J. Hazard. Mater. 2017, 322, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Fonseca, A.S.; Kuijpers, E.; Kling, K.I.; Levin, M.; Koivisto, A.J.; Nielsen, S.H.; Fransman, W.; Fedutik, Y.; Jensen, K.A.; Koponen, I.K. Particle release and control of worker exposure during laboratory-scale synthesis, handling and simulated spills of manufactured nanomaterials in fume hoods. J. Nanopart. Res. 2018, 20, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuhlbusch, T.A.J.; Wijnhoven, S.W.P.; Haase, A. Nanomaterial exposures for worker, consumer and the general public. NanoImpact 2018, 10, 11–25. [Google Scholar] [CrossRef]
- Jacobsen, N.R.; Stoeger, T.; van den Brule, S.; Saber, A.T.; Beyerle, A.; Vietti, G.; Mortensen, A.; Szarek, J.; Budtz, H.C.; Kermanizadeh, A.; et al. Acute and subacute pulmonary toxicity and mortality in mice after intratracheal instillation of ZnO nanoparticles in three laboratories. Food Chem. Toxicol. 2015, 85, 84–95. [Google Scholar] [CrossRef] [Green Version]
- Schwotzer, D.; Ernst, H.; Schaudien, D.; Kock, H.; Pohlmann, G.; Dasenbrock, C.; Creutzenberg, O. Effects from a 90-day inhalation toxicity study with cerium oxide and barium sulfate nanoparticles in rats. Part. Fibre Toxicol. 2017, 14, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Stefaniak, A.B.; Guilmette, R.A.; Day, G.A.; Hoover, M.D.; Breysse, P.N.; Scripsick, R.C. Characterization of phagolysosomal simulant fluid for study of beryllium aerosol particle dissolution. Toxicol. Vitr. 2005, 19, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Maraques, M.R.; Loebenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Keller, J.G.; Graham, U.M.; Koltermann-jülly, J.; Gelein, R.; Lan, M.; Landsiedel, R.; Wiemann, M.; Oberdörster, G.; Elder, A. Predicting dissolution and transformation of inhaled nanoparticles in the lung using abiotic flow cells: The case of barium sulfate. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Rozalen, M.; Ramos, M.E.; Gervilla, F.; Kerestedjian, T.; Fiore, S.; Huertas, F.J. Dissolution study of tremolite and anthophyllite: pH effect on the reaction kinetics. Appl. Geochem. 2014, 49, 46–56. [Google Scholar] [CrossRef]
- Berthelsen, R.; Klitgaard, M.; Rades, T.; Müllertz, A. In vitro digestion models to evaluate lipid based drug delivery systems; present status and current trends. Adv. Drug Deliv. Rev. 2019, 142, 35–49. [Google Scholar] [CrossRef]
- Plumlee, G.S.S.; Ziegler, T.L.L. The Medical Geochemistry of Dusts, Soils, and Other Earth Materials. Treatise on Geochemistry 2003, 9, 1–61. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD). Testing Programme of Manufactured Nanomaterials. Available online: https://www.oecd.org/chemicalsafety/nanosafety/testing-programme-manufactured-nanomaterials.htm (accessed on 28 September 2021).
- Clausen, P.A.; Kofoed-Sørensen, V.; Nørgaard, A.W.; Sahlgren, N.M.; Jensen, K.A. Thermogravimetry and mass spectrometry of extractable organics from manufactured nanomaterials for identification of potential coating components. Materials 2019, 12, 3657. [Google Scholar] [CrossRef] [Green Version]
- Jensen, K.A.; Kembouche, Y.; Christiansen, E.; Jacobsen, N.R.; Wallin, H.; Guiot, C.; Spalla, O.; Witschger, O. Towards a Method for Detecting the Potential Genotoxicity of Nanomaterials; Final protocol for producing suitable manufactured nanomaterial exposure media. The generic NANOGENOTOX dispersion protocol. Standard Operating Procedure (SOP) and background documentation; National Research Centre for the Working Environment: Copenhagen, Denmark, 2011; Available online: https://www.anses.fr/en/system/files/nanogenotox_deliverable_5.pdf (accessed on 12 December 2021).
- Malvern Instruments. Zeta potential: An Introduction in 30 minutes. Zetasizer Nano Serles Tech. Note. MRK654-01 2011, 2, 1–6. [Google Scholar]
- Fogler, H.S. Elements of Chemical Reaction Engineering, 3rd ed.; Pearson Education Limited: Hongkong, China, 1999; ISBN 9780135317167. [Google Scholar]
- Ionic Liquids Technologies GmbH. Technical Data Sheet Gamma-Aluminum Oxide Powder; Ionic Liquids Technologies GmbH: Heilbronn, Germany, 2019. [Google Scholar]
- De Temmerman, P.-J.; Mast, J.; Guiot, C.; Spalla, O.; Rousset, D.; Shivachev, B.; Tarassov, M.; Jensen, K.A. Towards a Method for detecting the Potential Genotoxicity of Nanomaterials; Nanogenotox deliverable 4.2: Transmission electron microscopic characterization of nanogenotox nanomaterials. 2012. Available online: https://www.anses.fr/en/system/files/nanogenotox_deliverable.2.pdf (accessed on 12 December 2021).
- Organisation for Economic Co-operation and Development (OECD). Dossier on Zinc Oxide. Series on the Safety of Manufactured Nanomaterials. No. 52; OECD: Paris, France, 2015; Available online: https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/JM/MONO(2015)15/ANN10&docLanguage=En (accessed on 12 December 2021).
- Rasmussen, K.; Mech, A.; Mast, J.; De Temmerman, P.; Waegeneers, N.; Van Steen, F.; Pizzolon, J.C.; De Temmerman, L.; Van Doren, E.; Jensen, A.; et al. Synthetic Amorphous Silicon Dioxide (NM-200, NM-201, NM-202, NM-203, NM-204): Characterisation and Physico-Chemical Properties, JRC Repository: NM-series of Representative Manufactured Nanomaterials. Eur. Sci. Tech. Res. Rep. 2013, 1–200. Available online: https://op.europa.eu/en/publication-detail/-/publication/7b93921d-e0be-4d73-beae-2061981861b2/language-en (accessed on 12 December 2021).
- Singh, C.; Friedrichs, S.; Ceccone, G.; Gibson, N.; Jensen, K.A.; Levin, M.; Goenaga-Infante, H.; Carlander, D.; Rasmussen, K. Cerium Dioxide NM-211, NM-212, NM-213, characterisation and test item preparation, JRC repository: NM-series of representative manufactured nanomaterials. Eur. Sci. Tech. Res. Rep. 2014, 1–88. Available online: https://op.europa.eu/en/publication-detail/-/publication/cb25df9a-c1db-4588-b4dc-6a42d9fceacb/language-en (accessed on 12 December 2021).
- Organisation for Economic Co-operation and Development (OECD). Dossier on Nanoclays. Series on the Safety of Manufactured Nanomaterials. No. 47; OECD: Paris, France, 2015; Available online: https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2015)10&doclanguage=en (accessed on 12 December 2021).
- Organisation for Economic Co-operation and Development (OECD). Titanium Dioxide: Summary of the Dossier. Series on the Safety of Manufactured Nanomaterials. No. 73. 2016. Available online: https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/JM/MONO(2016)25&docLanguage=En (accessed on 12 December 2021).
- Krause, B.; Meyer, T.; Sieg, H.; Kästner, C.; Reichardt, P.; Tentschert, J.; Jungnickel, H.; Estrela-Lopis, I.; Burel, A.; Chevance, S.; et al. Characterization of aluminum, aluminum oxide and titanium dioxide nanomaterials using a combination of methods for particle surface and size analysis. RSC Adv. 2018, 8, 14377–14388. [Google Scholar] [CrossRef] [Green Version]
- Singh, C.; Friedrichs, S.; Levin, M.; Birkedal, R.; Jensen, K.A.; Pojana, G.; Wohlleben, W.; Schulte, S.; Wiench, K.; Turney, T.; et al. Zinc Oxide NM-110, NM-111, NM-112, NM-113 Characterisation and Test Item Preparation, NM-Series of Representative Manufactured Nanomaterials. Eur. Sci. Tech. Res. Rep. 2011, 1–141. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC64075 (accessed on 12 December 2021).
- Rasmussen, K.; Mast, J.; De Temmerman, P.-J.; Verleysen, E.; Waegeneers, N.; Van Steen, F.; Pizzolon, J.C.; De Temmerman, L.; Van Doren, E.; Jensen, K.A.; et al. Titanium Dioxide, NM-100, NM-101, NM-102, NM-103, NM-104, NM-105: Characterisation and Physico- Chemical Properties. 2014. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC86291 (accessed on 12 December 2021).
- Pereira, E.I.; Minussi, F.B.; Cruz, C.C.T.; Bernardi, A.C.C.; Ribeiro, C.; Luiz, R.W.; Luiz, R.W. Urea − Montmorillonite-Extruded Nanocomposites: A Novel Slow- Release Material. J. Agric. Food Chem. 2012, 60, 5267–5272. [Google Scholar] [CrossRef]
- Jensen, K.A.; Pojana, G.; Bilanicova, D. Characterization of Manufactured Nanomaterials, Dispersion, and Exposure for Toxicological Testing, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Hartmann, N.B.; Jensen, K.A.; Baun, A.; Rasmussen, K.; Rauscher, H.; Tantra, R.; Cupi, D.; Gilliland, D.; Pianella, F.; Riego Sintes, J.M. Techniques and Protocols for Dispersing Nanoparticle Powders in Aqueous Media—Is there a Rationale for Harmonization? J. Toxicol. Environ. Health Part B 2015, 18, 299–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clogston, J.D.; Patri, A.K. Zeta Potential Measurement. In Characterization of Nanoparticles Intended for Drug Delivery; McNeil, S.E., Ed.; Humana Press: Totowa, NJ, USA, 2011; pp. 63–70. ISBN 978-1-60327-198-1. [Google Scholar]
- Mejia, J.; Unamur, S.L.; Booth, A.; Sintef, J.F.; Sabella, S.; Bove, P.; Iit, M.M.; Jensen, K.A.; Kembouche, Y.; Da Silva, E.; et al. NANoREG—Protocols for Exposure-Fate Characterization in Ecotoxicity and In Vitro Studies; National Institute for Public Health and the Environment: Utrecht, The Netherlands, 2016.
- Utembe, W.; Potgieter, K.; Stefaniak, A.B.; Gulumian, M. Dissolution and biodurability: Important parameters needed for risk assessment of nanomaterials. Part. Fibre Toxicol. 2015, 12, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, J.G.; Peijnenburg, W.; Werle, K.; Landsiedel, R.; Wohlleben, W. Understanding dissolution rates via continuous flow systems with physiologically relevant metal ion saturation in lysosome. Nanomaterials 2020, 10, 311. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Component | Chemical Formula | Concentration [mg/L] |
|---|---|---|
| Sodium phosphate dibasic anhydrous | Na2HPO4 | 142 |
| Sodium chloride | NaCl | 6650 |
| Sodium sulfate anhydrous | Na2SO4 | 71 |
| Calcium chloride dihydrate | CaCl2·2H2O | 29 |
| Glycine | H2NCH2CO2H | 450 |
| Potassium hydrogen phthalate | (1-(HO2C)-2-(CO2K)-C6H4) | 4085 |
| Alkylbenzyldimethylammonium chloride | - | 50 |
| Component | Chemical Formula | Concentration [mg/L] |
|---|---|---|
| Sodium chloride | NaCl | 6600 |
| Sodium bicarbonate | NaHCO3 | 2703 |
| Calcium chloride | CaCl2 | 22 |
| Sodium phosphate dibasic dodecahydrate | Na2HPO4·12H2O | 358 |
| Sodium sulfate anhydrous | Na2SO4 | 79 |
| Magnesium chloride hexahydrate | MgCl·6H2O | 212 |
| Glycine | H2NCH2CO2H | 118 |
| Sodium citrate dihydrate | Na3C6H5O7·2H2O | 153 |
| Sodium tartrate dihydrate | Na2C4H4O6·2H2O | 180 |
| Sodium pyruvate | C3H3NaO3 | 172 |
| Sodium lactate | C3H3NaO3 | 175 |
| Nanomaterial | Al2O3 | TiO2 NM-104 | ZnO NM-110 | ZnO NM-111 | ZnO NM-113 | SiO2 NM-200 | CeO2 NM-212 | Bentonite NM-600 |
|---|---|---|---|---|---|---|---|---|
| Phase | ɣ-Al2O3 | Rutile | Zincite | Zincite | Zincite | Synthetic amorphous silica | Cerianite | Montmorillonite, nanoclay |
| Specific surface area (SSA) [m2/g] | <200 a | 58.5 ± 46.3 b | 12.4 ± 0.6 c | 15.1 ± 0.6 c | 6.21 ± 0.4 c | 342 ± 36 d | 27.2 ± 0.9 e | 51.9 ± 1.6 f |
| Inorganic coating | - | Al2O3 | - | - | - | - | - | - |
| Organic coating | - | Glycerin g | - | Triethoxy- caprylsilane | - | - | - | - |
| Na2O [%] | 0.12 | - | - | - | 1.65 | - | 2.68 | |
| Al2O3 [%] | 102.17 | 6.08 | - | - | - | 0.94 | 0.75 | 17.57 |
| SiO2 [%] | 0.03 | 0.13 | - | 0.73 | - | 82.08 | 0.14 | 53.05 |
| P2O5 [%] | 0.0093 | - | - | - | - | 0.025 | - | 0.013 |
| SO3 [%] | 0.084 | 0.65 | - | - | 0.05 | 1.83 | 0.39 | 0.59 |
| Cl [%] | 0.014 | 0.03 | - | - | 0.02 | 0.11 | 0.13 | 0.14 |
| K2O [%] | - | - | - | - | 0.03 | - | 0.06 | |
| CaO [%] | 0.05 | - | - | - | 0.07 | - | 0.57 | |
| TiO2 [%] | 91.44 | 1.24 | 0.34 | 0.20 | 1.05 | - | 0.65 | |
| Fe2O3 [%] | 0.0034 | 0.01 | 0.01 | 0.007 | - | 0.04 | 0.08 | 4.62 |
| Ga2O3 [%] | - | - | - | - | - | - | 0.0044 | |
| CoO [%] | - | - | - | - | - | 0.03 | - | |
| NiO [%] | - | 0.007 | 0.007 | - | 0.0036 | - | - | |
| CuO [%] | 0.0032 | 0.006 | 0.04 | 0.03 | 0.04 | 0.01 | 0.04 | 0.0053 |
| ZnO [%] | - | 97.62 | 97.86 | 99.17 | 0.01 | 0.09 | 0.019 | |
| MgO [%] | - | - | - | - | 0.007 | 0.09 | 1.80 | |
| MnO [%] | - | - | - | - | - | - | 0.0066 | |
| ZrO2 [%] | 0.003 | - | - | - | 0.0067 | - | 0.017 | |
| MoO3 [%] | - | - | - | - | 0.0057 | - | - | |
| Nb2O5 [%] | 0.02 | - | - | - | - | - | 0.0025 | |
| CeO2 [%] | - | - | - | - | - | 97.70 | - | |
| Adsorbed moisture [%] | 3.77 | 1.50 ± 0.10 | 0.28 ± 0.11 | ND | 0.69 | 5.08 ± 0.12 | 0.13 | 6.63 |
| (n = 2) | (n = 3) | (n = 3) | (n = 3) | (n = 1) | (n = 3) | (n = 1) | (n = 1) | |
| LOI h [%] | ND | 3.11 ± 0.12 j | 0.59 ± 0.27 i,j | 1.59 ± 0.07 j | 0.20 j | 3.80 ± 0.13 | 0.71 | 5.29 k |
| (n = 1) | (n = 3) | (n = 3) | (n = 3) | (n = 1) | (n = 3) | (n = 1) | (n = 1) | |
| Total [%] | 106.09 | 103.13 | 99.77 | 100.57 | 100.37 | 96.75 | 100.28 | 93.72 |
| Test Medium | Gas Flow O2 [mL/min] | Gas Flow CO2 [mL/min] | Temperature [°C] | pH |
|---|---|---|---|---|
| Low-calcium Gamble’s solution | 144.4 ± 0.9 | 5.57 ± 0.23 | 36.7 ± 0.6 | 7.42 ± 0.14 |
| Phagolysosmal simulant fluid | 144.1 ± 0.1 | 5.59 ± 0.23 | 36.7 ± 0.3 | 4.48 ± 0.02 |
| Zave [nm] | PDI | ζpot [mV] | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Nanomaterial | I | II | III | I | II | III | I | II | III |
| Al2O3 | 184.8 ± 1.4 | 156.6 ± 1.4 | 165.7 ± 1.1 | 0.232 ± 0.009 | 0.162 ± 0.012 | 0.162 ± 0.013 | −21.28 ± 0.59 | −21.24 ± 1.28 | −22.37 ± 0.65 |
| TiO2 (NM-104) | 1027.2 ± 228.6 | 724.0 ± 160.2 | 1028.7 ± 468.7 | 0.805 ± 0.145 | 0.741 ± 0.140 | 0.719 ± 0.143 | −0.880 ± 0.241 | −0.840 ± 0.960 | 0.135 ± 0.302 |
| ZnO (NM-110) | 248.7 ± 2.9 | 247.7 ± 2.7 | 250.6 ± 1.1 | 0.146 ± 0.015 | 0.138 ± 0.016 | 0.138 ± 0.020 | −16.58 ± 0.44 | −14.21 ± 0.54 | −13.37 ± 0.27 |
| ZnO (NM-111) | 279.4 ± 2.7 | 283.5 ± 2.1 | 278.9 ± 2.9 | 0.148 ± 0.015 | 0.156 ± 0.020 | 0.155 ± 0.017 | −13.78 ± 0.84 | −14.72 ± 0.40 | −14.46 ± 0.54 |
| ZnO (NM-113) | 390.7 ± 5.0 | 402.1 ± 5.8 | 244.6 ± 5.8 | 0.206 ± 0.020 | 0.203 ± 0.020 | 0.229 ± 0.009 | −6.94 ± 0.49 | −6.27 ± 0.49 | −7.80 ± 1.01 |
| SiO2 (NM-200) | 4749.4 ± 773.0 | 4256.1 ± 991.5 | 2794 ±4 74.8 | 0.982 ± 0.053 | 0.982 ± 0.056 | 1.00 ± 0.00 | −38.21 ± 0.56 | −38.45 ± 0.64 | −39.20 ± 0.79 |
| CeO2 (NM-212) | 267.6 ± 4.6 | 259.6 ± 5.8 | 244.6 ± 5.8 | 0.220 ± 0.017 | 0.216 ± 0.015 | 0.218 ± 0.011 | 18.90 ± 0.76 | 25.81 ± 0.64 | 29.64 ± 0.93 |
| Bentonite (NM-600) | 246.3 ± 8.7 | 242.7 ± 4.1 | 242.2 ± 8.3 | 0.403 ± 0.040 | 0.368 ± 0.028 | 0.374 ± 0.030 | −43.05 ± 1.51 | −42.29 ± 1.42 | −44.08 ± 1.41 |
| Nanomaterial | Zave [nm] | ζpot [mV] | PDI |
|---|---|---|---|
| Al2O3 | 164.7 ± 1.3 | −23.52 ± 1.01 | 0.159 ± 0.016 |
| TiO2 (NM-104) | 366.7 ± 153.7 | −1.33 ± 1.36 | 0.304 ± 0.095 |
| ZnO (NM-110) | 247.1 ± 2.5 | −14.55 ± 0.58 | 0.145 ± 0.019 |
| ZnO (NM-111) | 275.9 ± 2.6 | −16.73 ± 0.80 | 0.147 ± 0.023 |
| ZnO (NM-113) | 375.8 ± 9.8 | −7.55 ± 0.69 | 0.205 ± 0.018 |
| SiO2 (NM-200) | 1985.9 ± 886.6 | −36.7 ± 0.7 | 0.945 ± 0.065 |
| CeO2 (NM-212) | 242.7 ± 4.2 | 18.52 ± 0.59 | 0.211 ± 0.017 |
| Bentonite (NM-600) | 253.6 ± 5.0 | −41.20 ± 1.04 | 0.352 ± 0.021 |
| Nanomaterial | Parallel | Equal |
|---|---|---|
| Al2O3 | 0.4149 | 0.6294 |
| TiO2 (NM-104), aluminum coating | 0.0597 | 0.7724 |
| ZnO (NM-110) | 0.1227 | 0.4823 |
| ZnO (NM-111) | <0.0001 | N/A |
| ZnO (NM-113) | 0.4184 | 0.9688 |
| SiO2 (NM-200) | 0.1080 | 0.8613 |
| CeO2 (NM-212) | 0.0021 | N/A |
| Bentonite (NM-600) | 0.0034 | N/A |
| Nanomaterial | Dissolution Rate,
Repeat I [mg/L/h] | Dissolution Rate,
Repeat II [mg/L/h] | Dissolution Rate,
Repeat III [mg/L/h] | Average of the Replicates within All Repeats, Surface Area Dissolution Rate (n = 9), [cm2/L/s] | Parallel, p-Value | Equal, p-Value |
|---|---|---|---|---|---|---|
| Al2O3 | 0.144 ± 0.080 | 0.090 ± 0.011 | 0.115 ± 0.047 | 0.065 ± 0.029 | 0.5277 | 0.4137 |
| TiO2 (NM-104), aluminum coating | 0.160 ± 0.038 | 0.159 ± 0.011 | 0.193 ± 0.022 | 0.027 ± 0.004 | 0.0074 | ND |
| ZnO (NM-110) | 2.04 ± 0.22 | 2.24 ± 0.83 | 5.42 ± 3.36 | 0.112 ± 0.082 | 0.6578 | <0.0001 |
| ZnO (NM-111) | 1.95 ± 0.26 | 1.50 ± 1.41 | 1.48 ± 0.61 | 0.074 ± 0.033 | 0.0627 | 0.0051 |
| ZnO (NM-113) | 1.73 ± 0.07 | 2.07 ± 0.50 | 2.38 ± 0.59 | 0.036 ± 0.008 | 0.4210 | 0.1727 |
| SiO2 (NM-200) | 3.09 ± 0.10 | 3.58 ± 0.13 | 2.92 ± 0.26 | 3.03 ± 0.317 | <0.0001 | ND |
| CeO2 (NM-212) | <LOD * | <LOD * | <LOD * | <LOD * | ND | ND |
| Bentonite (NM-600), release of silicon | 0.082 ± 0.028 | ND | 0.052 ± 0.020 | 0.096 ± 0.003 | 0.0052 | ND |
| Nanomaterial | Dissolution Rate,
[mg/L/h] | [cm2/L/s] |
|---|---|---|
| Al2O3 | 0.356 ± 0.001 | 0.197 ± 0.001 |
| TiO2 (NM-104), aluminum coating | 0.096 ± 0.002 | 0.015 ± 2.73 × 10−4 |
| ZnO (NM-110) | Highly soluble ⱡ | ND |
| ZnO (NM-111) | Highly soluble ⱡ | ND |
| ZnO (NM-113) | Highly soluble ⱡ | ND |
| SiO2 (NM-200) | 0.058 ± 3.29 × 10−3 | 0.055 ± 3.12 × 10−3 |
| CeO2 (NM-212) | 0.029 ± 5.03 × 10−3 | 2.20 × 10−3 ± 3.80 × 10−4 |
| Bentonite (NM-600), release of silicon | 0.059 ± 0.013 | 8.51 × 10−3 ± 1.91 × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holmfred, E.; Loeschner, K.; Sloth, J.J.; Jensen, K.A. Validation and Demonstration of an Atmosphere-Temperature-pH-Controlled Stirred Batch Reactor System for Determination of (Nano)Material Solubility and Dissolution Kinetics in Physiological Simulant Lung Fluids. Nanomaterials 2022, 12, 517. https://doi.org/10.3390/nano12030517
Holmfred E, Loeschner K, Sloth JJ, Jensen KA. Validation and Demonstration of an Atmosphere-Temperature-pH-Controlled Stirred Batch Reactor System for Determination of (Nano)Material Solubility and Dissolution Kinetics in Physiological Simulant Lung Fluids. Nanomaterials. 2022; 12(3):517. https://doi.org/10.3390/nano12030517
Chicago/Turabian StyleHolmfred, Else, Katrin Loeschner, Jens J. Sloth, and Keld Alstrup Jensen. 2022. "Validation and Demonstration of an Atmosphere-Temperature-pH-Controlled Stirred Batch Reactor System for Determination of (Nano)Material Solubility and Dissolution Kinetics in Physiological Simulant Lung Fluids" Nanomaterials 12, no. 3: 517. https://doi.org/10.3390/nano12030517
APA StyleHolmfred, E., Loeschner, K., Sloth, J. J., & Jensen, K. A. (2022). Validation and Demonstration of an Atmosphere-Temperature-pH-Controlled Stirred Batch Reactor System for Determination of (Nano)Material Solubility and Dissolution Kinetics in Physiological Simulant Lung Fluids. Nanomaterials, 12(3), 517. https://doi.org/10.3390/nano12030517