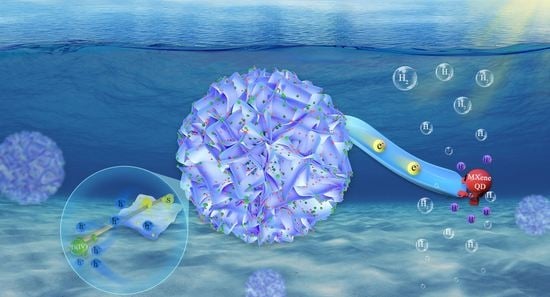
High-Stability Ti3C2-QDs/ZnIn2S4/Ti(IV) Flower-like Heterojunction for Boosted Photocatalytic Hydrogen Evolution
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Sample Preparation
2.2.1. Synthesis of ZIS Microspheres
2.2.2. Synthesis of MNSs and MQDs
2.2.3. Modification of ZIS by Amorphous Ti(IV) Co-Catalyst (ZIS/Ti(IV))
2.2.4. Modification of ZIS/Ti(IV) by MQDs Co-Catalyst (MQDs/ZIS/Ti(IV))
3. Results and Discussion
3.1. Characterization
3.2. Photocatalytic Properties
3.3. Mechanism Research
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gao, Y.; Xu, B.; Cherif, M.; Yu, H.; Zhang, Q.; Vidal, F.; Wang, X.; Ding, F.; Sun, Y.; Ma, D.; et al. Atomic insights for Ag interstitial/substitutional doping into ZnIn2S4 nanoplates and intimate coupling with reduced graphene oxide for enhanced photocatalytic hydrogen production by water splitting. Appl. Catal. B 2020, 279, 119403. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Peng, Y.; Hu, C.; Chen, Z. Self-assembled synthesis of benzene-ring-grafted g-C3N4 nanotubes for enhanced photocatalytic H2 evolution. Appl. Catal. B 2020, 279, 119401. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 photocatalysis: Concepts, mechanisms, and challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, Y.H.; Li, X.; Zhang, Q.; Yu, H.; Peng, X.; Peng, F. Regulating electron-hole separation to promote photocatalytic H2 evolution activity of nanoconfined Ru/MXene/TiO2 catalysts. ACS Nano 2020, 14, 14181–14189. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, S.; Cao, Y.; Tao, Y.; Li, X.; Pan, D.; Phillips, D.L.; Zhang, D.; Chen, M.; Li, G.; et al. Edge-enriched ultrathin MoS2 embedded yolk-shell TiO2 with boosted charge transfer for superior photocatalytic H2 evolution. Adv. Funct. Mater. 2019, 29, 1901958. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, C.; Zuo, G.; Guo, Y.; Xiao, W.; Dai, Y.; Kong, J.; Xu, X.; Zhou, Y.; Xie, A.; et al. 0D/2D Co3O4/TiO2 Z-Scheme heterojunction for boosted photocatalytic degradation and mechanism investigation. Appl. Catal. B 2020, 278, 119298. [Google Scholar] [CrossRef]
- Pan, X.; Shang, C.; Chen, Z.; Jin, M.; Zhang, Y.; Zhang, Z.; Wang, X.; Zhou, G. Enhanced photocatalytic H2 evolution over ZnIn2S4 flower-like microspheres doped with black phosphorus quantum dots. Nanomaterials 2019, 9, 1266. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Li, Y.; Zhao, P.; Zhang, L.; Dai, B.; Xu, J.; Huang, H.; He, Y.; Leung, D.Y.C. Novel Z-scheme Ag-C3N4/SnS2 plasmonic heterojunction photocatalyst for degradation of tetracycline and H2 production. Chem. Eng. J. 2021, 405, 126555. [Google Scholar] [CrossRef]
- Wang, K.; Xing, Z.; Meng, D.; Zhang, S.; Li, Z.; Pan, K.; Zhou, W. Hollow MoSe2@Bi2S3/CdS Core-Shell Nanostructure as Dual Z-Scheme Heterojunctions with Enhanced Full Spectrum Photocatalytic-Photothermal Performance. Appl. Catal. B 2021, 281, 119482. [Google Scholar] [CrossRef]
- Sun, B.; Zhou, W.; Li, H.; Ren, L.; Qiao, P.; Li, W.; Fu, H. Synthesis of Particulate Hierarchical Tandem Heterojunctions toward Optimized Photocatalytic Hydrogen Production. Adv. Mater. 2018, 30, 1804282. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Shang, Q.; Wang, Y.; Jiao, L.; Yao, T.; Li, Y.; Zhang, Q.; Luo, Y.; Jiang, H.L. Single Pt atoms confined into a metal-organic framework for efficient photocatalysis. Adv. Mater. 2018, 30, 1705112. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Chen, Z.; Shui, L.; Zhou, G.; Wang, X. Novel 2D/2D BiOBr/UMOFNs direct Z-scheme photocatalyst for efficient phenol degradation. Nanotechnology 2021, 32, 045711. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Tong, L.; Yi, X.; Zhou, W.; Wang, D.; Liu, L.; Ye, J. Ultrathin graphene encapsulated Cu nanoparticles: A highly stable and efficient catalyst for photocatalytic H2 evolution and degradation of isopropanol. Chem. Eng. J. 2020, 390, 124558. [Google Scholar] [CrossRef]
- Hu, J.; Chen, C.; Zheng, Y.; Zhang, G.; Guo, C.; Li, C.M. Spatially separating redox centers on Z-Scheme ZnIn2S4/BiVO4 hierarchical heterostructure for highly efficient photocatalytic hydrogen evolution. Small 2020, 16, 2002988. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Guan, B.Y.; Lou, X.W.D. Construction of ZnIn2S4-In2O3 hierarchical tubular heterostructures for efficient CO2 photoreduction. J. Am. Chem. Soc. 2018, 140, 5037–5040. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, Z.; Li, S.; Su, Y.; Huang, L.; Shao, N.; Liu, F.; Bu, Y.; Zhang, H.; Zhang, Z. Role of SnS2 in 2D–2D SnS2/TiO2 Nanosheet Heterojunctions for Photocatalytic Hydrogen Evolution. ACS Appl. Nano Mater. 2019, 2, 2144–2151. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Ao, D. Construction of heterostructured ZnIn2S4@NH2-MIL-125(Ti) nanocomposites for visible-light-driven H2 production. Appl. Catal. B 2018, 221, 433–442. [Google Scholar] [CrossRef]
- Khan, S.; Choi, H.; Kim, D.; Lee, S.Y.; Zhu, Q.; Zhang, J.; Kim, S.; Cho, S.-H. Self-assembled heterojunction of metal sulfides for improved photocatalysis. Chem. Eng. J. 2020, 395, 125092. [Google Scholar] [CrossRef]
- Zuo, G.; Wang, Y.; Teo, W.L.; Xian, Q.; Zhao, Y. Direct Z-scheme TiO2–ZnIn2S4 nanoflowers for cocatalyst-free photocatalytic water splitting. Appl. Catal. B 2021, 291, 120126. [Google Scholar] [CrossRef]
- Du, C.; Zhang, Q.; Lin, Z.; Yan, B.; Xia, C.; Yang, G. Half-unit-cell ZnIn2S4 monolayer with sulfur vacancies for photocatalytic hydrogen evolution. Appl. Catal. B 2019, 248, 193–201. [Google Scholar] [CrossRef]
- Wen, Y.; Rufford, T.E.; Chen, X.; Li, N.; Lyu, M.; Dai, L.; Wang, L. Nitrogen-doped Ti3C2Tx MXene electrodes for high-performance supercapacitors. Nano Energy 2017, 38, 368–376. [Google Scholar] [CrossRef]
- Kshetri, T.; Tran, D.T.; Le, H.T.; Nguyen, D.C.; Hoa, H.V.; Kim, N.H.; Lee, J.H. Recent advances in MXene-based nanocomposites for electrochemical energy storage applications. Prog. Mater. Sci. 2020, 117, 100733. [Google Scholar] [CrossRef]
- Shahzad, F.; Iqbal, A.; Kim, H.; Koo, C.M. 2D transition metal carbides (MXenes): Applications as an electrically conducting material. Adv. Mater. 2020, 32, 2002159. [Google Scholar] [CrossRef]
- Zhou, J.; Zha, X.; Zhou, X.; Chen, F.; Gao, G.; Wang, S.; Shen, C.; Chen, T.; Zhi, C.; Eklund, P.; et al. Synthesis and electrochemical properties of two-dimensional hafnium carbide. ACS Nano 2017, 11, 3841–3850. [Google Scholar] [CrossRef] [Green Version]
- Ming, F.; Liang, H.; Huang, G.; Bayhan, Z.; Alshareef, H.N. MXenes for rechargeable batteries beyond the lithium-ion. Adv. Mater. 2020, 33, 2004039. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Guo, X.; Wu, W.; Wang, G. 2D metal carbides and nitrides (MXenes) as high-performance electrode materials for lithium-based batteries. Adv. Energy Mater. 2018, 8, 1801897. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Ren, A.; Wu, J.; Wang, Z. Recent advances in 2D MXenes for photodetection. Adv. Funct. Mater. 2020, 30, 2000907. [Google Scholar] [CrossRef]
- Kim, S.J.; Koh, H.J.; Ren, C.E.; Kwon, O.; Maleski, K.; Cho, S.Y.; Anasori, B.; Kim, C.K.; Choi, Y.K.; Kim, J.; et al. Metallic Ti3C2Tx MXene gas sensors with ultrahigh signal-to-noise ratio. ACS Nano 2018, 12, 986–993. [Google Scholar] [CrossRef] [Green Version]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th anniversary article: MXenes: A new family of two-dimensional materials. Adv. Energy Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Chen, X.; Ong, W.-J.; Zhao, X.; Li, N. Surface and heterointerface engineering of 2D MXenes and their nanocomposites: Insights into electro- and photocatalysis. Chem 2019, 5, 18–50. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Zhang, N. Positioning MXenes in the photocatalysis landscape: Competitiveness, challenges, and future perspectives. Adv. Funct. Mater. 2020, 30, 2002528. [Google Scholar] [CrossRef]
- Zuo, G.; Wang, Y.; Teo, W.L.; Xie, A.; Guo, Y.; Dai, Y.; Zhou, W.; Jana, D.; Xian, Q.; Dong, W.; et al. Enhanced photocatalytic water oxidation by hierarchical 2D-Bi2MoO6@2D-MXene Schottky junction nanohybrid. Chem. Eng. J. 2021, 403, 126328. [Google Scholar] [CrossRef]
- Sun, Y.; Meng, X.; Dall’Agnese, Y.; Dall’Agnese, C.; Duan, S.; Gao, Y.; Chen, G.; Wang, X.-F. 2D MXenes as co-catalysts in photocatalysis: Synthetic methods. Nano-Micro Lett. 2019, 11, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ran, J.; Gao, G.; Li, F.T.; Ma, T.Y.; Du, A.; Qiao, S.Z. Ti3C2 MXene co-catalyst on metal sulfide photo-absorbers for enhanced visible-light photocatalytic hydrogen production. Nat. Commun. 2017, 8, 13907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, L.; Chen, Q.; Li, J.; Liu, H. Boosting the photocatalytic activity of CdLa2S4 for hydrogen production using Ti3C2 MXene as a co-catalyst. Appl. Catal. B 2020, 267, 118379. [Google Scholar] [CrossRef]
- Cai, T.; Wang, L.; Liu, Y.; Zhang, S.; Dong, W.; Chen, H.; Yi, X.; Yuan, J.; Xia, X.; Liu, C.; et al. Ag3PO4/Ti3C2 MXene interface materials as a Schottky catalyst with enhanced photocatalytic activities and anti-photocorrosion performance. Appl. Catal. B 2018, 239, 545–554. [Google Scholar] [CrossRef]
- Du, X.; Zhao, T.; Xiu, Z.; Xing, Z.; Li, Z.; Pan, K.; Yang, S.; Zhou, W. BiVO4@ZnIn2S4/Ti3C2 MXene quantum dots assembly all-solid-state direct Z-Scheme photocatalysts for efficient visible-light-driven overall water splitting. Appl. Mater. Today 2020, 20, 100719. [Google Scholar] [CrossRef]
- Xiao, R.; Zhao, C.; Zou, Z.; Chen, Z.; Tian, L.; Xu, H.; Tang, H.; Liu, Q.; Lin, Z.; Yang, X. In situ fabrication of 1D CdS nanorod/2D Ti3C2 MXene nanosheet Schottky heterojunction toward enhanced photocatalytic hydrogen evolution. Appl. Catal. B 2020, 268, 118382. [Google Scholar] [CrossRef]
- Zuo, G.; Wang, Y.; Teo, W.L.; Xie, A.; Guo, Y.; Dai, Y.; Zhou, W.; Jana, D.; Xian, Q.; Dong, W.; et al. Ultrathin ZnIn2S4 nanosheets anchored on Ti3C2TX MXene for photocatalytic H2 evolution. Angew. Chem. Int. Ed. 2020, 59, 11287–11292. [Google Scholar] [CrossRef]
- Li, Y.; Ding, L.; Guo, Y.; Liang, Z.; Cui, H.; Tian, J. Boosting the photocatalytic ability of g-C3N4 for hydrogen production by Ti3C2 MXene quantum dots. ACS Appl. Mater. Interfaces 2019, 11, 41440–41447. [Google Scholar] [CrossRef] [PubMed]
- Cavdar, O.; Malankowska, A.; Amgar, D.; Mazierski, P.; Łuczak, J.; Lisowski, W.; Zaleska-Medynska, A. Remarkable visible-light induced hydrogen generation with ZnIn2S4 microspheres/CuInS2 quantum dots photocatalytic system. Int. J. Hydrogen Energy 2021, 46, 486–498. [Google Scholar] [CrossRef]
- Janani, R.; Sumathi, S.; Gupta, B.; Shaheer, A.R.M.; Ganapathy, S.; Neppolian, B.; Roy, S.C.; Channakrishnappa, R.; Paul, B.; Singh, S. Development of CdTe quantum dot supported ZnIn2S4 hierarchical microflowers for improved photocatalytic activity. J. Environ. Chem. Eng. 2022, 10, 107030. [Google Scholar] [CrossRef]
- Gao, F.; Zhao, Y.; Zhang, L.; Wang, B.; Wang, Y.; Huang, X.; Wang, K.; Feng, W.; Liu, P. Well Dispersed MoC Quantum Dots in Ultrathin Carbon Film as Efficient Co-catalyst for Photocatalytic H2 Evolution. J. Mater. Chem. A 2018, 6, 18979–18986. [Google Scholar] [CrossRef]
- Liu, M.; Inde, R.; Nishikawa, M.; Qiu, X.; Atarashi, D.; Sakai, E.; Nosaka, Y.; Hashimoto, K.; Miyauchi, M. Enhanced photoactivity with nanocluster-grafted titanium dioxide photocatalysts. ACS Nano 2014, 8, 7229–7238. [Google Scholar] [CrossRef]
- Hu, S.; Shaner, M.R.; Beardslee, J.A.; Lichterman, M.; Brunschwig, B.S.; Lewis, N.S. Amorphous TiO2 coatings stabilize Si, GaAs, and GaP photoanodes for efficient water oxidation. Science 2014, 344, 1005–1009. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Chen, W.; Wang, X.; Xu, Y.; Yu, J. Enhanced photocatalytic activity and photoinduced stability of Ag-based photocatalysts: The synergistic action of amorphous-Ti(IV) and Fe(III) cocatalysts. Appl. Catal. B 2016, 187, 163–170. [Google Scholar] [CrossRef]
- Ma, G.; Shang, C.; Jin, M.; Shui, L.; Meng, Q.; Zhang, Y.; Zhang, Z.; Liao, H.; Li, M.; Chen, Z.; et al. Amorphous Ti(IV)-modified flower-like ZnIn2S4 microspheres with enhanced hydrogen evolution photocatalytic activity and simultaneous wastewater purification. J. Mater. Chem. C 2020, 8, 2693–2699. [Google Scholar] [CrossRef]
- Ding, L.; Li, D.; Shen, H.; Qiao, X.; Shen, H.; Shi, W. 2D β-NiS as electron harvester anchors on 2D ZnIn2S4 for boosting photocatalytic hydrogen production. J. Alloys Compd. 2021, 853, 157328. [Google Scholar] [CrossRef]
- Uddin, A.; Muhmood, T.; Guo, Z.; Gu, J.; Chen, H.; Jiang, F. Hydrothermal synthesis of 3D/2D heterojunctions of ZnIn2S4/oxygen doped g-C3N4 nanosheet for visible light driven photocatalysis of 2,4-dichlorophenoxyacetic acid degradation. J. Alloys Compd. 2020, 845, 156206. [Google Scholar] [CrossRef]
- Cao, Y.; Xing, Z.; Li, Z.; Wu, X.; Hu, M.; Yan, X.; Zhu, Q.; Yang, S.; Zhou, W. Mesoporous black TiO2-x/Ag nanospheres coupled with g-C3N4 nanosheets as 3D/2D ternary heterojunctions visible light photocatalysts. J. Hazard. Mater. 2018, 343, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Kant, S.; Pathania, D.; Singh, P.; Dhiman, P.; Kumar, A. Removal of malachite green and methylene blue by Fe0.01Ni0.01Zn0.98O/polyacrylamide nanocomposite using coupled adsorption and photocatalysis. Appl. Catal. B 2014, 147, 340–352. [Google Scholar] [CrossRef]
- Chen, X.; Xu, W.; Ding, N.; Ji, Y.; Pan, G.; Zhu, J.; Zhou, D.; Wu, Y.; Chen, C.; Song, H. Dual interfacial modification engineering with 2D MXene quantum dots and copper sulphide nanocrystals enabled high-performance perovskite solar cells. Adv. Funct. Mater. 2020, 30, 2003295. [Google Scholar] [CrossRef]
- Zhou, J.; Tian, G.; Chen, Y.; Meng, X.; Shi, Y.; Cao, X.; Pan, K.; Fu, H. In situ controlled growth of ZnIn2S4 nanosheets on reduced graphene oxide for enhanced photocatalytic hydrogen production performance. Chem. Commun. 2013, 49, 2237. [Google Scholar] [CrossRef]
- Cao, S.; Shen, B.; Tong, T.; Fu, J.; Yu, J. 2D/2D Heterojunction of Ultrathin MXene/Bi2WO6 Nanosheets for Improved Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2018, 28, 1800136. [Google Scholar] [CrossRef]
- Näslund, L.-Å.; Persson, P.O.Å.; Rosen, J. X-ray Photoelectron Spectroscopy of Ti3AlC2, Ti3C2Tz, and TiC Provides Evidence for the Electrostatic Interaction between Laminated Layers in MAX-Phase Materials. J. Phys. Chem. C 2020, 124, 27732–27742. [Google Scholar] [CrossRef]
- Zeng, Z.; Yan, Y.; Chen, J.; Zan, P.; Tian, Q.; Chen, P. Boosting the photocatalytic ability of Cu2O nanowires for CO2 conversion by MXene quantum dots. Adv. Funct. Mater. 2019, 29, 1806500. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Chen, Z.; Wang, X.; Jin, M. High-Stability Ti3C2-QDs/ZnIn2S4/Ti(IV) Flower-like Heterojunction for Boosted Photocatalytic Hydrogen Evolution. Nanomaterials 2022, 12, 542. https://doi.org/10.3390/nano12030542
Yang L, Chen Z, Wang X, Jin M. High-Stability Ti3C2-QDs/ZnIn2S4/Ti(IV) Flower-like Heterojunction for Boosted Photocatalytic Hydrogen Evolution. Nanomaterials. 2022; 12(3):542. https://doi.org/10.3390/nano12030542
Chicago/Turabian StyleYang, Liqin, Zhihong Chen, Xin Wang, and Mingliang Jin. 2022. "High-Stability Ti3C2-QDs/ZnIn2S4/Ti(IV) Flower-like Heterojunction for Boosted Photocatalytic Hydrogen Evolution" Nanomaterials 12, no. 3: 542. https://doi.org/10.3390/nano12030542
APA StyleYang, L., Chen, Z., Wang, X., & Jin, M. (2022). High-Stability Ti3C2-QDs/ZnIn2S4/Ti(IV) Flower-like Heterojunction for Boosted Photocatalytic Hydrogen Evolution. Nanomaterials, 12(3), 542. https://doi.org/10.3390/nano12030542