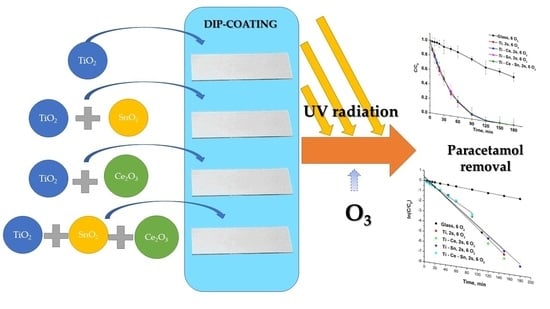
Removal of Paracetamol from Aqueous Solutions by Photocatalytic Ozonation over TiO2-MexOy Thin Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Sol-Gel Synthesis
2.3. Preparation of Thin Films
2.4. Thin Films Characterization
2.5. Photoreactor, Photocatalytic Activity, and Aqueous Effluents Analysis
3. Results and Discussions
3.1. Thin Film Characterization
3.2. Photocatalytic Ozonation Tests
3.2.1. Ozone Consumption
3.2.2. Kinetic Study
3.2.3. Photocatalytic Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Santos, L.; Araújo, A.N.; Fachini, A.; Pena, A.; Delerue-Matos, C.; Montenegro, M. Ecotoxicological Aspects Related to the Presence of Pharmaceuticals in the Aquatic Environment. J. Hazard. Mat. 2009, 175, 45–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persson, M.; Sabelström, E.; Gunnarsson, B. Handling of unused prescription drugs—Knowledge, behaviour and attitude among Swedish people. Environ. Int. 2008, 35, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Yang, M.Y.; Hu, J.; Ren, L.; Zhang, Y.; Li, K. Determination and fate of oxytetracycline and related compounds in oxytetracycline production wastewater and the receiving river. Environ. Toxicol. Chem. 2008, 27, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Gomez Ramos, M.J.; Petrovic, M.; Fernández-Alba, A.; Barcelo, D. Determination of Pharmaceuticals of Various Therapeutic Classes by Solid-Phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry Analysis in Hospital Effluent Wastewaters. J. Chromatogr. A 2006, 1114, 224–233. [Google Scholar] [CrossRef]
- Aitken, M.; Kleinrock, M. Global Medicines Use in 2020. Outlook and Implications. Available online: https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/global-medicines-use-in-2020 (accessed on 18 December 2021).
- Hughes, S.; Kay, P.; Brown, L. Global Synthesis and Critical Evaluation of Pharmaceutical Data Sets Collected from River Systems. Environ. Sci. Technol. 2012, 47, 661–677. [Google Scholar] [CrossRef]
- Braun, P.; Lange, F.; Schrader, S.; Lorenz, W. Determination of Endocrine Disrupting Compounds and Acidic Drugs in Water by Coupling of Derivatization, Gas Chromatography and Negative-Chemical Ionization Mass Spectrometry. Clean 2007, 35, 444–451. [Google Scholar] [CrossRef]
- Cleuvers, M. Mixture Toxicity of the Anti-Inflammatory Drugs Diclofenac, Ibuprofen, Naproxen, and Acetylsalicylic Acid. Ecotoxicol. Environ. Saf. 2004, 59, 309–315. [Google Scholar] [CrossRef]
- RFDTV, PAP and Paracetamol Market Size In 2021: Top Countries Data with 2.0% CAGR, Global Industry Brief Analysis by Top Key companies and Growth Insights to 2027. Latest 136 Pages Report. Available online: https://www.rfdtv.com/story/44670147/pap-and-paracetamol-market-size-in-2021-top-countries-data-with-20-cagr-global-industry-brief-analysis-by-top-key-companies-and-growth-insights-to (accessed on 19 December 2021).
- Al-Kaf, A.G.; Naji, K.M.; Abdullah, Q.Y.M.; Edrees, W.H.A. Occurrence of Paracetamol in Aquatic Environments and Transformation by Microorganisms: A Review. Chron. Pharm. Sci. 2017, 1, 341–355. [Google Scholar]
- Zhang, Z.; Lin, F.; Xiang, L.; Yu, H.; Wang, Z.; Yan, B.; Chen, G. Synergistic effect for simultaneously catalytic ozonation of chlorobenzene and NO over MnCoO catalysts: Byproducts formation under practical conditions. Chem. Eng. J. 2022, 427, 130929. [Google Scholar] [CrossRef]
- Yuan, Y.; Garg, S.; Wang, Y.; Li, W.; Chen, G.; Gao, M.; Zhong, J.; Wang, J.; Waite, T.D. Influence of salinity on the heterogeneous catalytic ozonation process: Implications to treatment of high salinity wastewater. J. Hazard. Mat. 2022, 423, 127255. [Google Scholar] [CrossRef]
- Wang, Z.; Xian, W.; Ma, Y.; Xu, T.; Jiang, R.; Zhu, H.; Mao, X. Catalytic ozonation with disilicate-modified nZVI for quinoline removal in aqueous solution: Efficiency and heterogeneous reaction mechanism. Sep. Purif. Technol. 2022, 281, 119961. [Google Scholar] [CrossRef]
- Shao, S.; Li, Z.; Gao, K.; Zhang, J.; Liu, Y.; Jiao, W. Preparation of Cu-MnOX/γ-Al2O3 by high gravity-assisted impregnation method for heterogeneous catalytic ozonation of nitrobenzene. Sep. Purif. Technol. 2022, 280, 119896. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Moussavi, G.; Oulego, P.; Giannakis, S. Heterogeneous catalytic ozonation and peroxone-mediated removal of Acetaminophen using natural and modified hematite-rich soil, as efficient and environmentally friendly catalysts. Appl. Catal. B Environ. 2022, 301, 120786. [Google Scholar] [CrossRef]
- Heidari, Z.; Pelalak, R.; Eshaghi Malekshah, R.; Pishnamazi, M.; Rezakazemi, M.; Aminabhavi, T.M.; Shirazian, S. A new insight into catalytic ozonation of sulfasalazine antibiotic by plasma-treated limonite nanostructures: Experimental, modeling and mechanism. Chem. Eng. J. 2022, 428, 131230. [Google Scholar] [CrossRef]
- Guan, Z.; Guo, Y.; Huang, Z.; Liao, X.; Chen, S.; Ou, X.; Sun, S.; Liang, J.; Cai, Y.; Xie, W.; et al. Simultaneous and efficient removal of organic Ni and Cu complexes from electroless plating effluent using integrated catalytic ozonation and chelating precipitation process in a continuous pilot-scale system. Chem. Eng. J. 2022, 428, 131250. [Google Scholar] [CrossRef]
- Zuo, X.; Ma, S.; Wu, Q.; Xiong, J.; He, J.; Ma, C.; Chen, Z. Nanometer CeO2 doped high silica ZSM-5 heterogeneous catalytic ozonation of sulfamethoxazole in water. J. Hazard. Mater. 2021, 411, 125072. [Google Scholar] [CrossRef]
- Zhang, Z.; Ai, H.; Fu, M.-L.; Hu, Y.-b.; Liu, J.; Ji, Y.; Vasanthakumar, V.; Yuan, B. A new insight into catalytic ozonation of ammonia by MgO/Co3O4 composite: The effects, reaction kinetics and mechanism. Chem. Eng. J. 2021, 418, 129461. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, B.; Shan, C.; Zhang, W.; Dionysiou, D.D.; Pan, B. Roles of oxygen-containing functional groups of O-doped g-C3N4 in catalytic ozonation: Quantitative relationship and first-principles investigation. Appl. Catal. B Environ. 2021, 292, 120155. [Google Scholar] [CrossRef]
- Zhang, F.; Liao, J.; Lu, J.; Niu, J. Extensive incorporation of carboxyl groups into g-C3N4 by integrated oxygen doping and HNO3 oxidation for enhanced catalytic ozonation of para-chlorobenzoic acid and atrazine. Sep. Purif. Technol. 2021, 256, 117806. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, Y.; Li, W.; Geng, J.; Ren, H.; Xu, K. Mechanism and toxicity evaluation of catalytic ozonation over Cu/Ce-Al2O3 system aiming at degradation of humic acid in real wastewater. Sci. Rep. 2021, 11, 8748. [Google Scholar] [CrossRef]
- Sun, Z.; Ma, J.; Liu, Y.; Wang, H.; Cao, W.; Zhu, N.; Lou, Z. Mineralization of refractory organics in oil refinery wastewater by the catalytic ozonation with magnetic praseodymium-catalysts: Catalytic performances and mechanisms. Sep. Purif. Technol. 2021, 277, 119506. [Google Scholar] [CrossRef]
- Suligoj, A.; Kete, M.; Cernigoj, U.; Fresno, F.; Lavrencic Stangar, U. Synergism in TiO2 photocatalytic ozonation for the removal of dichloroacetic acid and thiacloprid. Environ. Res. 2021, 197, 110982. [Google Scholar] [CrossRef]
- Song, J.; Ma, N.; Chen, W.; Chen, J.; Dai, Q. Insights into mechanism of catalytic ozonation of cinnamyl alcohol over core-shell Fe3O4@SiO2@La2O3 catalyst. Sep. Purif. Technol. 2021, 282, 119969. [Google Scholar] [CrossRef]
- Ren, H.; Wang, Z.; Chen, X.; Jing, Z.; Qu, Z.; Huang, L. Effective mineralization of p-nitrophenol by catalytic ozonation using Ce-substituted La1xCexFeO3 catalyst. Chemosphere 2021, 285, 131473. [Google Scholar] [CrossRef]
- Rashidashmagh, F.; Doekhi-Bennani, Y.; Tizghadam-Ghazani, M.; van der Hoek, J.P.; Mashayekh-Salehi, A.; Heijman, B.; Yaghmaeian, K. Synthesis and characterization of SnO2 crystalline nanoparticles: A new approach for enhancing the catalytic ozonation of acetaminophen. J. Hazard. Mater. 2021, 404, 124154. [Google Scholar] [CrossRef]
- Psaltou, S.; Kaprara, E.; Triantafyllidis, K.; Mitrakas, M.; Zouboulis, A. Heterogeneous catalytic ozonation: The significant contribution of PZC value and wettability of the catalysts. J. Environ. Chem. Eng. 2021, 9, 106173. [Google Scholar] [CrossRef]
- Mu, J.; Li, S.; Wang, J.; Li, X.; Chen, W.; Tong, X.; Tang, Y.; Li, L. Efficient catalytic ozonation of bisphenol A by three-dimensional mesoporous CeOx-loaded SBA-16. Chemosphere 2021, 278, 130412. [Google Scholar] [CrossRef]
- Meng, F.; Guo, L.; He, J.; Wang, Z.; Ma, Z.; Zeng, Y.; Zhang, S.; Zhong, Q. V2O5-(NH4)2V6O16·1.5H2O composite catalysts as novel platforms for high-efficiency catalytic ozonation of NO under low temperature. J. Phys. Chem. Solids 2021, 155, 110112. [Google Scholar] [CrossRef]
- Zhao, Y.; An, H.; Dong, G.; Feng, J.; Ren, Y.; Wei, T. Elevated removal of di-n-butyl phthalate by catalytic ozonation over magnetic Mn-doped ferrospinel ZnFe2O4 materials: Efficiency and mechanism. Appl. Surf. Sci. 2020, 505, 144476. [Google Scholar] [CrossRef]
- Zhang, H.; He, Y.; Lai, L.; Yao, G.; Lai, B. Catalytic ozonation of Bisphenol A in aqueous solution by Fe3O4–MnO2 magnetic composites: Performance, transformation pathways and mechanism. Sep. Purif. Technol. 2020, 245, 116449. [Google Scholar] [CrossRef]
- Chávez, A.M.; Solís, R.R.; Beltrán, F.J. Magnetic graphene TiO2-based photocatalyst for the removal of pollutants of emerging concern in water by simulated sunlight aided photocatalytic ozonation. Appl. Catal. B Environ. 2020, 262, 118275. [Google Scholar] [CrossRef]
- Chávez, A.M.; Quiñones, D.H.; Rey, A.; Beltrán, F.J.; Álvarez, P.M. Simulated solar photocatalytic ozonation of contaminants of emerging concern and effluent organic matter in secondary effluents by a reusable magnetic catalyst. Chem. Eng. J. 2020, 398, 125642. [Google Scholar] [CrossRef]
- Asgari, E.; Farzadkia, M.; Esrafili, A.; Badi, M.Y.; Jokandan, S.F.; Sobhi, H.R. Application of a photocatalytic ozonation process using TiO2 magnetic nanoparticles for the removal of Ceftazide from aqueous solutions: Evaluation of performance, comparative study and mechanism. Optik 2020, 212, 164667. [Google Scholar] [CrossRef]
- Orge, C.A.; Soares, O.S.G.P.; Ramalho, P.S.F.; Pereira, M.F.R.; Faria, J.L. Magnetic Nanoparticles for Photocatalytic Ozonation of Organic Pollutants. Catalysts 2019, 9, 703. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, M.A.; Abidin, K.; Eliyana, A.; Malago, J.D.; Noor, F.A.; Winata, T. Nickel thin film preparation and its characterization as catalyst for HWC-in plasma-PECVD-growth graphene. Mat. Today Proc. 2021, 44, 3420–3425. [Google Scholar] [CrossRef]
- Hosseini, A.; Karimi, H.; Foroughi, J.; Sabzehmeidani, M.M.; Ghaedi, M. Heterogeneous photoelectro-Fenton using ZnO and TiO2 thin film as photocatalyst for photocatalytic degradation Malachite Green. Appl. Surf. Sci. Adv. 2021, 6, 100126. [Google Scholar] [CrossRef]
- Zhang, X.; Ptasinska, S. Dissociative adsorption of H2O onto a Pt thin film in direct contact with GaN (0001): Effect of electronic communications between catalyst and a semiconducting support. Appl. Surf. Sci. 2020, 516, 146127. [Google Scholar] [CrossRef]
- Mauraya, A.K.; Mahana, D.; Pal, P.; Muthiah, S.; Singh, P.; Muthusamy, S.K. Effect of bulk and surface modification of SnO2 thin films with PdO catalyst on CO gas sensing characteristics prepared by vacuum evaporation process. J. Alloys Compd. 2020, 843, 155979. [Google Scholar] [CrossRef]
- Sankarasubramanian, K.; Babu, K.J.; Soundarrajan, P.; Logu, T.; Gnanakumar, G.; Ramamurthi, K.; Sethuraman, K.; Senthil Kumar, S.M. A new catalyst Ti doped CdO thin film for non-enzymatic hydrogen peroxide sensor application. Sens. Actuators B Chem. 2019, 285, 164–172. [Google Scholar] [CrossRef]
- Tyagi, P.; Sharma, A.; Tomar, M.; Gupta, V. SnO2 thin film sensor having NiO catalyst for detection of SO2 gas with improved response characteristics. Sens. Actuators B Chem. 2017, 248, 998–1005. [Google Scholar] [CrossRef]
- Li, Y.; Song, W.; Liu, J.; Zhao, Z.; Gao, M.; Wei, Y.; Wang, Q.; Deng, J. The protection of CeO2 thin film on Cu-SAPO-18 catalyst for highly stable catalytic NH3-SCR performance. Chem. Eng. J. 2017, 330, 926–935. [Google Scholar] [CrossRef]
- Fatahpour, M.; Noori Sadeh, F.; Hazeri, N.; Maghsoodlou, M.T.; Hadavi, M.S.; Mahnaei, S. Ag/TiO2 nano-thin films as robust heterogeneous catalyst for one-pot, multi-component synthesis of bis (pyrazol-5-ol) and dihydropyrano[2,3-c]pyrazole analogs. J. Saudi Chem. Soc. 2017, 21, 998–1006. [Google Scholar] [CrossRef]
- Kim, S.; Chang, H.-K.; Kim, K.B.; Kim, H.-J.; Lee, H.-N.; Park, T.J.; Park, Y.M. Highly Porous SnO2/TiO2 Heterojunction Thin-Film Photocatalyst Using Gas-Flow Thermal Evaporation and Atomic Layer Deposition. Catalysts 2021, 11, 1144. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, X.; Liu, C.; Qian, X.; Wen, Y.; Yang, Q.; Sun, T.; Chang, W.; Liu, X.; Chen, Z. Facile Construction of All-Solid-State Z-Scheme g-C3N4/TiO2 Thin Film for the Efficient Visible-Light Degradation of Organic Pollutant. Nanomaterials 2020, 10, 600. [Google Scholar] [CrossRef] [Green Version]
- Nicosia, A.; Vento, F.; Di Mari, G.M.; D’Urso, L.; Mineo, P.G. TiO2-Based Nanocomposites Thin Film Having Boosted Photocatalytic Activity for XenobioticsWater Pollution Remediation. Nanomaterials 2021, 11, 400. [Google Scholar] [CrossRef]
- Stathatos, E.; Lianos, P.; Tsakiroglou, C. Highly efficient nanocrystalline titania films made from organic/inorganic nanocomposite gels. Micropor. Mesopor. Mat. 2004, 75, 255–260. [Google Scholar] [CrossRef]
- Zheng, Z.; Ng, Y.H.; Tang, Y.; Li, Y.; Chen, W.; Wang, J.; Li, X.; Li, L. Visible-light-driven photoelectrocatalytic activation of chloride by nanoporous MoS2@BiVO4 photoanode for enhanced degradation of bisphenol A. Chemosphere 2020, 263, 128279. [Google Scholar] [CrossRef]
- Pinar Gokdemir, F.; Ece Yuzbasioglu, V.; Keskin, B.; Ozdemir, O.; Kutlu, K. Formation of TiO2 Thin Films by a Modified Sol-gel Route and Characterization of Structural, Optical and Electrochromic Properties. Adv. Mat. Lett. 2014, 5, 367–371. [Google Scholar] [CrossRef]
- Haider, A.J.; Thamir, A.D.; Najim, A.A.; Ali, G.A. Improving Efficiency of TiO2:Ag/Si Solar Cell Prepared by Pulsed Laser Deposition. Plasmonics 2016, 12, 105–115. [Google Scholar] [CrossRef]
- Thamaphat, K.; Limsuwan, P.; Ngotawornchai, B. Phase characterization of TiO2 powder by XRD and TEM. Kasetsart J. 2008, 42, 357–361. [Google Scholar]
- Ba-Abbad, M.; Kadhum, A.; Mohamad, A.B.; Takriff, M.; Sopian, K. Synthesis and Catalytic Activity of TiO2 Nanoparticles for Photochemical Oxidation of Concentrated Chlorophenols under Direct Solar Radiation. Int. J. Electrochem. Sci. 2012, 7, 4871–4888. [Google Scholar]
- Avciata, O.; Benli, Y.; Gördük, S.; Gördük, Ö. Ag doped TiO2 nanoparticles prepared by hydrothermal method and coating of the nanoparticles on the ceramic pellets for photocatalytic study: Surface properties and photoactivity. J. Eng. Technol. Appl. Sci. 2016, 1, 34–50. [Google Scholar] [CrossRef]
- Wei, C.H.; Tang, X.H.; Liang, J.R.; Tan, S.Y. Preparation, characterization and photocatalytic activities of boron- and cerium-codoped TiO2. J. Environ. Sci. 2007, 19, 90–96. [Google Scholar] [CrossRef]
- Hassan, M.S.; Amna, T.; Al-Deyab, S.S.; Kim, H.C.; Oh, T.H.; Khil, M.S. Toxicity of Ce2O3/TiO2 composite nanofibers against S. aureus and S. typhimurium: A novel electrospun material for disinfection of food pathogens. Colloids Surf. A Physicochem. Eng. Asp. 2012, 415, 268–273. [Google Scholar] [CrossRef]
- Patel, S.B.; Radevski, N.; Mondinos, N.; Zhao, X.; Jack, K.; Veder, J.P.; Zhou, Z.F.; Jiang, Z.T. A mechanical and modelling study of magnetron sputtered cerium-titanium oxide film coatings on Si (100). Ceram. Int. 2019, 45, 6875–6884. [Google Scholar] [CrossRef]
- Duan, N.; Lin, H.; Li, L.; Hu, J.; Bi, L.; Lu, H.; Weng, X.; Xie, J.; Deng, L. ZrO2-TiO2 thin films: A new material system for mid-infrared integrated photonics. Opt. Mater. Express 2013, 3, 1537–1545. [Google Scholar] [CrossRef]
- Lubas, M.; Jasinski, J.J.; Sitarz, M.; Kurpaska, L.; Podsiad, P.; Jasinski, J. Raman spectroscopy of TiO2 thin films formed by hybrid treatment for biomedical applications. Spectrochim Acta A Mol. Biomol. Spectrosc. 2014, 133, 867–871. [Google Scholar] [CrossRef]
- Balzano, V.; Cavaliere, E.; Fanetti, M.; Gardonio, S.; Gavioli, L. The Role of Substrate on Thermal Evolution of Ag/TiO2 Nanogranular Thin Films. Nanomaterials 2021, 11, 2253. [Google Scholar] [CrossRef]
- Medjaldi, F.; Bouabellou, A.; Bouachiba, Y.; Taabouche, A.; Bouatia, K.; Serrar, H. Study of TiO2, SnO2 and nanocomposites TiO2:SnO2 thin films prepared by sol-gel method: Successful elaboration of variable–refractive index systems. Mat. Res. Express 2020, 7, 016439. [Google Scholar] [CrossRef]
- Topuz, B.B.; Gündüz, G.; Mavis, B.; Çolak, Ü. The effect of tin dioxide (SnO2) on the anatase-rutile phase transformation of titania (TiO2) in mica-titania pigments and their use in paint. Dyes Pigm. 2011, 90, 123–128. [Google Scholar] [CrossRef]
- Mosquera, A.A.; Albella, J.M.; Navarro, V.; Bhattacharyya, D.; Endrino, J.L. Effect of silver on the phase transition and wettability of titanium oxide films. Sci. Rep. 2016, 6, 32171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabir, I.; Sheppard, L.; Shhamiri, R.; Koshy, P.; Liu, R.; Joe, W.; Le, A.; Lu, X.; Chen, W.F.; Sorrell, C. Correction to: Contamination of TiO2 thin films spin coated on borosilicate and rutile substrates. J. Mat. Sci. 2021, 56, 18341–18343. [Google Scholar] [CrossRef]
- Singhal, A.; Ebrahimi, F.; Hosseini, S. A comprehensive review on the modeling of smart piezoelectric structures. Struct. Eng. Mech. 2020, 74, 611–633. [Google Scholar]
- Olkun, A.; Pat, S.; Akkurt Özgür, N.; Mohammadigharehbagh, R.; Demirkol, U.; Özgür, M.; Korkmaz, Ş. Detailed transmittance analysis of high-performance SnO2-doped WO3 thin films in UV–Vis region for electrochromic devices. J. Mat. Sci. Mat. Electron. 2020, 27, 19074–19084. [Google Scholar] [CrossRef]
- Yoneyama, H.; Haga, S.; Yamanaka, S. Photocatalytic activities of microcrystalline titania incorporated in sheet silicates of clay. J. Phys. Chem. 1989, 93, 4833–4837. [Google Scholar] [CrossRef]
- Kabir, I.I.; Sheppard, L.R.; Shahmiri, R.; Liu, R.; Le, A.; Lu, X.; Hanaor, D.; Chen, W.F.; Koshy, P.; Sorrell, C.C. Correction to: Contamination of TiO2 thin films spin coated on rutile and soda–lime–silica substrates. J. Mat. Sci. 2021, 56, 17874–17875. [Google Scholar] [CrossRef]
- Zhao, J.; Cao, J.; Zhao, Y.; Zhang, T.; Zheng, D.; Li, C. Catalytic ozonation treatment of papermaking wastewater by Ag-doped NiFe2O4: Performance and mechanism. J. Environ. Sci. 2020, 97, 75–84. [Google Scholar] [CrossRef]
- Xu, B.; Zhou, J.L.; Altaee, A.; Ahmed, M.B.; Johir, M.A.H.; Ren, J.; Li, X. Improved photocatalysis of perfluorooctanoic acid in water and wastewater by Ga2O3/UV system assisted by peroxymonosulfate. Chemosphere 2020, 239, 124722. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.; Chen, B.; Bu, Y.; Chen, Y.; Ma, J.; Rosario-Ortiz, F.L. Photolysis and photocatalysis of haloacetic acids in water: A review of kinetics, influencing factors, products, pathways, and mechanisms. J. Hazard. Mater. 2020, 391, 122143. [Google Scholar] [CrossRef]
- Tan, C.; Wu, H.; He, H.; Lu, X.; Gao, H.; Deng, J.; Chu, W. Anti-inflammatory drugs degradation during LED-UV365 photolysis of free chlorine: Roles of reactive oxidative species and formation of disinfection by-products. Water Res. 2020, 185, 116252. [Google Scholar] [CrossRef]
- Park, K.Y.; Yu, Y.J.; Yun, S.J.; Kweon, J.H. Natural organic matter removal from algal-rich water and disinfection by-products formation potential reduction by powdered activated carbon adsorption. J. Environ. Manag. 2019, 235, 310–318. [Google Scholar] [CrossRef]
- Majumder, A.; Gupta, B.; Gupta, A.K. Pharmaceutically active compounds in aqueous environment: A status, toxicity and insights of remediation. Environ. Res. 2019, 176, 108542. [Google Scholar] [CrossRef]
- Sorlini, S. Natural Organic Matter: Characterization and Removal by AOPs to Assist Drinking Water Facilities. In Applications of Advanced Oxidation Processes (AOPs) in Drinking Water Treatment; Gil, A., Galeano, L.A., Vicente, M.A., Eds.; Springer: Cham, Switzerland, 2017; pp. 53–68. [Google Scholar]
- Ghosh, D.; Mondal, S.; Ramakrishna, K. Pharmacobotanical, physicochemical and phytochemical characterisation of a rare salt-secreting mangrove Aegialitis rotundifolia Roxb., (Plumbaginaceae) leaves: A comprehensive pharmacognostical study. South Afr. J. Bot. 2017, 113, 212–229. [Google Scholar] [CrossRef]
- Ganiyu, S.O.; Oturan, N.; Raffy, S.; Cretin, M.; Causserand, C.; Oturan, M.A. Efficiency of plasma elaborated sub-stoichiometric titanium oxide (Ti4O7) ceramic electrode for advanced electrochemical degradation of paracetamol in different electrolyte media. Sep. Purif. Technol. 2019, 208, 142–152. [Google Scholar] [CrossRef] [Green Version]
- Zur, J.; Pinski, A.; Marchlewicz, A.; Hupert-Kocurek, K.; Wojcieszynska, D.; Guzik, U. Organic micropollutants paracetamol and ibuprofen-toxicity, biodegradation, and genetic background of their utilization by bacteria. Environ. Sci. Pollut. Res. 2018, 25, 21498–21524. [Google Scholar] [CrossRef] [Green Version]
- Lopez Zavala, M.A.; Jaber Lara, C.R. Degradation of Paracetamol and Its Oxidation Products in Surface Water by Electrochemical Oxidation. Environ. Eng. Sci. 2018, 35, 1248–1254. [Google Scholar] [CrossRef] [Green Version]
- Villota, N.; Lomas, J.M.; Camarero, L.M. Study of the paracetamol degradation pathway that generates color and turbidity in oxidized wastewaters by photo-Fenton technology. J. Photochem. Photobiol. A Chem. 2016, 329, 113–119. [Google Scholar] [CrossRef]
- Neamtu, M.; Bobu, M.; Kettrup, A.; Siminiceanu, I. Ozone photolysis of paracetamol in aqueous solution. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2013, 48, 1264–1271. [Google Scholar] [CrossRef]
- Costamagna, P.; Delucchi, M.; Busca, G.; Giordano, A. System for ammonia removal from anaerobic digestion and associated ammonium sulfate production: Simulation and design considerations. Proc. Safet. Environ. Protect. 2020, 144, 133–142. [Google Scholar] [CrossRef]
- Liu, F.; Sun, L.; Wan, J.; Tang, A.; Deng, M.; Wu, R. Organic matter and ammonia removal by a novel integrated process of constructed wetland and microbial fuel cells. RSC Adv. 2019, 9, 5384–5393. [Google Scholar] [CrossRef] [Green Version]
- Kang, W.; Chen, S.; Yu, H.; Xu, T.; Wu, S.; Wang, X.; Lu, N.; Quan, X.; Liang, H. Photocatalytic ozonation of organic pollutants in wastewater using a flowing through reactor. J. Hazard. Mater. 2021, 405, 124277. [Google Scholar] [CrossRef]
- Chen, X.; Zhan, S.; Chen, D.; He, C.; Tian, S.; Xiong, Y. Grey Fe-CeO2-σ for boosting photocatalytic ozonation of refractory pollutants: Roles of surface and bulk oxygen vacancies. Appl. Catal. B Environ. 2021, 286, 119928. [Google Scholar] [CrossRef]
- Asgari, E.; Sheikhmohammadi, A.; Nourmoradi, H.; Nazari, S.; Aghanaghad, M. Degradation of ciprofloxacin by photocatalytic ozonation process under irradiation with UVA: Comparative study, performance and mechanism. Process Saf. Environ. Prot. 2021, 147, 356–366. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, D.; Lu, C.; Dai, S.; Li, J.; Guan, W.; Li, D. Enhanced photocatalytic ozonation of 2,4-dichlorophenoxyacetic acid using P25/g-C3N4 composite under visible light irradiation. Desalination Water Treat. 2020, 208, 377–385. [Google Scholar] [CrossRef]
- Orge, C.A.; Sampaio, M.J.; Faria, J.L.; Pereira, M.F.R.; Silva, C.G. Efficiency and stability of metal-free carbon nitride in the photocatalytic ozonation of oxamic acid under visible light. J. Environ. Chem. Eng. 2020, 8, 104172. [Google Scholar] [CrossRef]
- Bahadori, E.; Conte, F.; Tripodi, A.; Ramis, G.; Rossetti, I. Photocatalytic Selective Oxidation of Ammonia in a Semi-Batch Reactor: Unravelling the Effect of Reaction Conditions and Metal Co-Catalysts. Catalysts 2021, 11, 209. [Google Scholar] [CrossRef]
- Joudi, F.; Naceur, J.B.; Ouertani, R.; Chtourou, R. A novel strategy to produce compact and adherent thin films of SnO2/TiO2 composites suitable for water splitting and pollutant degradation. J. Mater. Sci. Mat. Electron. 2018, 30, 167–179. [Google Scholar] [CrossRef]
- Jiang, D.; Otitoju, T.A.; Ouyang, Y.; Shoparwe, N.F.; Wang, S.; Zhang, A.; Li, S. A Review on Metal Ions Modified TiO2 for Photocatalytic Degradation of Organic Pollutants. Catalysts 2021, 11, 1039. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Liu, F.; Li, M.; Qi, X.; Xue, M.; Wang, Y.; Han, F. Construction of CeO2/TiO2 heterojunctions immobilized on activated carbon fiber and its synergetic effect between adsorption and photodegradation for toluene removal. J. Nanopart. Res. 2020, 22, 122. [Google Scholar] [CrossRef]















| Sample | RMS Roughness (nm) |
|---|---|
| (Ti) | 1.1 |
| (Ti-Ce) | 5.9 |
| (Ti-Sn) | 2.97 |
| (Ti-Ce-Sn) | 2.1 |
| Sample | Band Gap |
|---|---|
| (Ti) | 3.68 |
| (Ti-Ce) | 3.44 |
| (Ti-Sn) | 3.68 |
| (Ti-Ce-Sn) | 3.38 |
| Catalytic System/Ozone Concentration | Consumed Ozone (mg) | Residual Ozone (mg) | Ozone Input (mg) | mgO3/mg TOC Removed (Consumed Ozone) | mgO3/mg TOC Removed (Ozone Input) |
|---|---|---|---|---|---|
| Glass, 12 O3 | 261.38 | 98.62 | 360 | 54.10 | 74.51 |
| Ti, 4s, 12 O3 | 341.85 | 18.15 | 360 | 48.88 | 51.48 |
| Ti-Ce, 4s, 12 O3 | 254.86 | 105.14 | 360 | 36.44 | 51.48 |
| Ti-Sn, 4s, 12 O3 | 290.01 | 69.99 | 360 | 47.52 | 58.98 |
| Ti-Ce-Sn, 4s, 12 O3 | 286.53 | 73.47 | 360 | 39.53 | 49.67 |
| Ti, 2s, 12 O3 | 324.45 | 35.55 | 360 | 45.57 | 50.56 |
| Ti-Ce, 2s, 12 O3 | 343.54 | 16.46 | 360 | 67.54 | 70.78 |
| Ti-Sn, 2s, 12 O3 | 327.26 | 32.74 | 360 | 77.99 | 85.80 |
| Ti-Ce-Sn, 2s, 12 O3 | 342.22 | 17.78 | 360 | 61.17 | 64.35 |
| Glass, 6 O3 | 157.34 | 22.66 | 180 | 412.47 | 471.88 |
| Ti, 2s, 6 O3 | 160.72 | 19.28 | 180 | 33.26 | 37.25 |
| Ti-Ce, 2s, 6 O3 | 164.79 | 15.21 | 180 | 40.50 | 44.24 |
| Ti-Sn, 2s, 6 O3 | 155.56 | 24.44 | 180 | 33.98 | 39.32 |
| Ti-Ce-Sn, 2s, 6 O3 | 167.28 | 12.72 | 180 | 54.82 | 58.98 |
| Catalytic System/Ozone Concentration | kobs (min−1) 102 | R2 |
|---|---|---|
| Glass, 12 O3 | 4.07 | 0.9913 |
| Ti, 4s, 12 O3 | 6.12 | 0.98778 |
| Ti-Ce, 4s, 12 O3 | 5.37 | 0.99401 |
| Ti-Sn, 4s, 12 O3 | 6.26 | 0.99085 |
| Ti-Ce-Sn, 4s, 12 O3 | 5.19 | 0.98929 |
| Ti, 2s, 12 O3 | 3.90 | 0.98754 |
| Ti-Ce, 2s, 12 O3 | 4.97 | 0.99081 |
| Ti-Sn, 2s, 12 O3 | 4.51 | 0.98938 |
| Ti-Ce-Sn, 2s, 12 O3 | 4.91 | 0.9672 |
| Glass, 6 O3 | 0.23 | 0.99029 |
| Ti, 2s, 6 O3 | 4.04 | 0.97132 |
| Ti-Ce, 2s, 6 O3 | 4.35 | 0.95859 |
| Ti-Sn, 2s, 6 O3 | 3.99 | 0.98008 |
| Ti-Ce-Sn, 2s, 6 O3 | 2.87 | 0.98847 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avramescu, S.M.; Fierascu, I.; Fierascu, R.C.; Brazdis, R.I.; Nica, A.V.; Butean, C.; Olaru, E.A.; Ulinici, S.; Verziu, M.N.; Dumitru, A. Removal of Paracetamol from Aqueous Solutions by Photocatalytic Ozonation over TiO2-MexOy Thin Films. Nanomaterials 2022, 12, 613. https://doi.org/10.3390/nano12040613
Avramescu SM, Fierascu I, Fierascu RC, Brazdis RI, Nica AV, Butean C, Olaru EA, Ulinici S, Verziu MN, Dumitru A. Removal of Paracetamol from Aqueous Solutions by Photocatalytic Ozonation over TiO2-MexOy Thin Films. Nanomaterials. 2022; 12(4):613. https://doi.org/10.3390/nano12040613
Chicago/Turabian StyleAvramescu, Sorin Marius, Irina Fierascu, Radu Claudiu Fierascu, Roxana Ioana Brazdis, Angel Vasile Nica, Claudia Butean, Elena Alina Olaru, Sorin Ulinici, Marian Nicolae Verziu, and Anca Dumitru. 2022. "Removal of Paracetamol from Aqueous Solutions by Photocatalytic Ozonation over TiO2-MexOy Thin Films" Nanomaterials 12, no. 4: 613. https://doi.org/10.3390/nano12040613
APA StyleAvramescu, S. M., Fierascu, I., Fierascu, R. C., Brazdis, R. I., Nica, A. V., Butean, C., Olaru, E. A., Ulinici, S., Verziu, M. N., & Dumitru, A. (2022). Removal of Paracetamol from Aqueous Solutions by Photocatalytic Ozonation over TiO2-MexOy Thin Films. Nanomaterials, 12(4), 613. https://doi.org/10.3390/nano12040613