Environmental Fate and Toxicity of Sunscreen-Derived Inorganic Ultraviolet Filters in Aquatic Environments: A Review
Abstract
:1. Introduction
2. Inorganic UVFs in Aquatic Environments
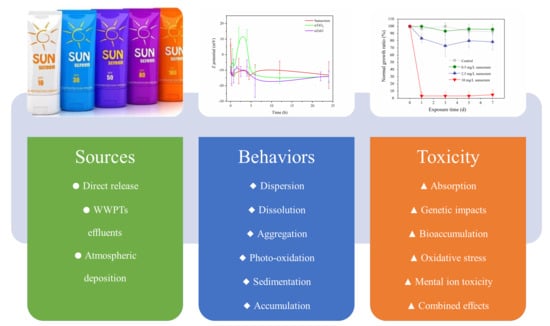
2.1. Sources and Occurrences
2.2. Environmental Behaviors
2.3. Substantial Environmental Impacts
3. Toxicity of Inorganic UVFs on Aquatic Organisms
3.1. Interaction of Inorganic UVFs with Organisms in Aquatic Environments
3.2. Toxicity of Inorganic UVFs on Organisms at the Individual Level
3.2.1. nTiO2 UVFs
3.2.2. nZnO UVFs
3.3. Impacts of Inorganic UVFs on Multiple Trophic Levels
3.4. Potential Mechanisms for the Toxicity of Sunscreen-Derived Inorganic UVFs
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanchez-Quiles, D.; Tovar-Sanchez, A. Are sunscreens a new environmental risk associated with coastal tourism? Environ. Int. 2015, 83, 158–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, H.W.; Draelos, Z.D. Clinical Guide to Sunscreens and Photoprotection; Informa Health Care: New York, NY, USA, 2008. [Google Scholar]
- Urbach, F. The historical aspects of sunscreens. J. Photochem. Photobiol. B Biol. 2001, 64, 99–104. [Google Scholar]
- Tovar-Sanchez, A.; Sanchez-Quiles, D.; Basterretxea, G.; Benede, J.L.; Chisvert, A.; Salvador, A.; Moreno-Garrido, I.; Blasco, J. Sunscreen products as emerging pollutants to coastal waters. PLoS ONE 2013, 8, e65451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gormsen, E. The impact of tourism on coastal areas. GeoJournal 1997, 42, 39–54. [Google Scholar] [CrossRef]
- Li, W.; Ma, Y.; Guo, C.; Hu, W.; Liu, K.; Wang, Y.; Zhu, T. Occurrence and behavior of four of the most used sunscreen UV filters in a wastewater reclamation plant. Water Res. 2007, 41, 3506–3512. [Google Scholar] [CrossRef]
- Honey, M.; Krantz, D. Global Trends in Coastal Tourism; Center on Ecotourism and Sustainable Development: Washington, DC, USA, 2007. [Google Scholar]
- Hall, C.M. Trends in ocean and coastal tourism: The end of the last frontier? Ocean. Coast. Manag. 2001, 44, 601–618. [Google Scholar] [CrossRef]
- Sanchez-Quiles, D.; Tovar-Sanchez, A. Sunscreens as a source of hydrogen peroxide production in coastal waters. Environ. Sci. Technol. 2014, 48, 9037–9042. [Google Scholar] [CrossRef] [Green Version]
- Manová, E.; von Goetz, N.; Hauri, U.; Bogdal, C.; Hungerbühler, K. Organic UV filters in personal care products in Switzerland: A survey of occurrence and concentrations. Int. J. Hyg. Environ. Health 2013, 216, 508–514. [Google Scholar] [CrossRef]
- Giokas, D.L.; Salvador, A.; Chisvert, A. UV filters: From sunscreens to human body and the environment. TrAC Trends Anal. Chem. 2007, 26, 360–374. [Google Scholar] [CrossRef]
- Salvador, A.; Chisvert, A. Analysis of Cosmetic Products; Elsevier Science: Amsterdam, The Netherlands; London, UK, 2011. [Google Scholar]
- Zhu, X.S.; Huang, J.Y.; Lü, X.H.; Du, Y.F.; Cai, Z.H. Fate and Toxicity of UV Filters in Marine Environments. Huanjing Kexue/Environ. Sci. 2018, 39, 2991–3002. [Google Scholar]
- Environmental Working Group. EWG’s Sunscreen Guide. 2019. Available online: https://www.ewg.org/sunscreen/report/executive-summary/ (accessed on 20 December 2020).
- Antoniou, C.; Kosmadaki, M.G.; Stratigos, A.J.; Katsambas, A.D. Sunscreens–what’s important to know. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1110–1119. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.-Y.; Zhu, B.S.; Wang, X.-F.; Lu, Q.H. Cytotoxicity of titanium dioxide nanoparticles in mouse fibroblast cells. Chem. Res. Toxicol. 2008, 21, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Seite, S.; Colige, A.; Piquemal-Vivenot, P.; Montastier, C.; Fourtanier, A.; Lapiere, C.; Nusgens, B. A full-UV spectrum absorbing daily use cream protects human skin against biological changes occurring in photoaging. Photodermatol. Photoimmunol. Photomed. 2000, 16, 147–155. [Google Scholar] [CrossRef]
- van der Pols, J.C.; Williams, G.M.; Pandeya, N.; Logan, V.; Green, A.C. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiol. Prev. Biomark. 2006, 15, 2546–2548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balogh, T.S.; Velasco, M.; Pedriali, C.A.; Kaneko, T.M.; Baby, A.R. Ultraviolet radiation protection: Current available resources in photoprotection. BrasDermatol 2011, 86, 732–742. [Google Scholar]
- Wang, S.Q.; Balagula, Y.; Osterwalder, U. Photoprotection: A review of the current and future technologies. Dermatol. Ther. 2010, 23, 31–47. [Google Scholar] [CrossRef]
- Schlumpf, M.; Cotton, B.; Conscience, M.; Haller, V.; Steinmann, B.; Lichtensteiger, W. In vitro and in vivo estrogenicity of UV screens. Environ. Health Perspect. 2001, 109, 239–244. [Google Scholar] [CrossRef]
- Schneider, S.L.; Lim, H.W. A review of inorganic UV filters zinc oxide and titanium dioxide. Photodermatol. Photoimmunol. Photomed. 2019, 35, 442–446. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.J.; Huang, S.C.; Chen, Y.P.; Chiueh, L.C.; Shih, D.Y.C. Analysis of titanium dioxide and zinc oxide nanoparticles in cosmetics. J. Food Drug Anal. 2015, 23, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Watkinson, A.C.; Bunge, A.L.; Hadgraft, J.; Lane, M.E. Nanoparticles do not penetrate human skin-a theoretical perspective. Pharm. Res. 2013, 30, 1943–1946. [Google Scholar] [CrossRef]
- Piccinno, F.; Gottschalk, F.; Seeger, S.; Nowack, B. Industrial production quantities and uses of ten engineered nanomaterials in Europe and the world. J. Nanoparticle Res. 2012, 14, 1109. [Google Scholar] [CrossRef] [Green Version]
- Danovaro, R.; Bongiorni, L.; Corinaldesi, C.; Giovannelli, D.; Damiani, E.; Astolfi, P.; Greci, L.; Pusceddu, A. Sunscreens cause coral bleaching by promoting viral infections. Environ. Health Perspect. 2008, 116, 441–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewicka, Z.A.; Benedetto, A.F.; Benoit, D.N.; William, W.Y.; Fortner, J.D.; Colvin, V.L. The structure, composition, and dimensions of TiO2 and ZnO nanomaterials in commercial sunscreens. J. Nanoparticle Res. 2011, 13, 3607. [Google Scholar] [CrossRef]
- Botta, C.; Labille, J.; Auffan, M.; Borschneck, D.; Miche, H.; Cabié, M.; Masion, A.; Rose, J.; Bottero, J.-Y. TiO2-based nanoparticles released in water from commercialized sunscreens in a life-cycle perspective: Structures and quantities. Environ. Pollut. 2011, 159, 1543–1550. [Google Scholar] [CrossRef]
- Tsui, M.M.; Leung, H.; Wai, T.-C.; Yamashita, N.; Taniyasu, S.; Liu, W.; Lam, P.K.; Murphy, M.B. Occurrence, distribution and ecological risk assessment of multiple classes of UV filters in surface waters from different countries. Water Res. 2014, 67, 55–65. [Google Scholar] [CrossRef]
- Jurado, A.; Gago-Ferrero, P.; Vàzquez-Suñé, E.; Carrera, J.; Pujades, E.; Díaz-Cruz, M.S.; Barceló, D. Urban groundwater contamination by residues of UV filters. J. Hazard. Mater. 2014, 271, 141–149. [Google Scholar] [CrossRef]
- Amine, H.; Gomez, E.; Halwani, J.; Casellas, C.; Fenet, H. UV filters, ethylhexyl methoxycinnamate, octocrylene and ethylhexyl dimethyl PABA from untreated wastewater in sediment from eastern Mediterranean river transition and coastal zones. Mar. Pollut. Bull. 2012, 64, 2435–2442. [Google Scholar] [CrossRef]
- Barón, E.; Gago-Ferrero, P.; Gorga, M.; Rudolph, I.; Mendoza, G.; Zapata, A.M.; Díaz-Cruz, S.; Barra, R.; Ocampo-Duque, W.; Páez, M. Occurrence of hydrophobic organic pollutants (BFRs and UV-filters) in sediments from South America. Chemosphere 2013, 92, 309–316. [Google Scholar] [CrossRef]
- Rodil, R.; Moeder, M. Development of a simultaneous pressurised-liquid extraction and clean-up procedure for the determination of UV filters in sediments. Anal. Chim. Acta 2008, 612, 152–159. [Google Scholar] [CrossRef]
- Rainieri, S.; Barranco, A.; Primec, M.; Langerholc, T. Occurrence and toxicity of musks and UV filters in the marine environment. Food Chem. Toxicol. 2017, 104, 57–68. [Google Scholar]
- Díaz-Cruz, M.S.; Barceló, D. Chemical analysis and ecotoxicological effects of organic UV-absorbing compounds in aquatic ecosystems. TrAC Trends Anal. Chem. 2009, 28, 708–717. [Google Scholar] [CrossRef]
- Balmer, M.E.; Buser, H.R.; Muller, M.D.; Poiger, T. Occurrence of some organic UV filters in wastewater, in surface waters, and in fish from Swiss lakes. Environ. Sci. Technol. 2005, 39, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Poiger, T.; Buser, H.R.; Balmer, M.E.; Bergqvist, P.A.; Muller, M.D. Occurrence of UV filter compounds from sunscreens in surface waters: Regional mass balance in two Swiss lakes. Chemosphere 2004, 55, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.W.; Leung, P.T.; Djurisic, A.B.; Leung, K.M. Toxicities of nano zinc oxide to five marine organisms: Influences of aggregate size and ion solubility. Anal. Bioanal. Chem. 2010, 396, 609–618. [Google Scholar] [CrossRef]
- Gondikas, A.P.; Kammer, F.; Reed, R.B.; Wagner, S.; Ranville, J.F.; Hofmann, T. Release of TiO2 nanoparticles from sunscreens into surface waters: A one-year survey at the old Danube recreational Lake. Environ. Sci. Technol. 2014, 48, 5415–5422. [Google Scholar] [CrossRef]
- SCCNFP, Opinion Concerning Titanium Dioxide. Opinion: European Commission—The Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers. Sci. Comm. Cosmet. Prod. Non-Food Prod. Intend. Consum. 2000, 0005/98, 2–43. Available online: https://ec.europa.eu/health/ph_risk/committees/sccp/documents/out135_en.pdf (accessed on 15 November 2021).
- Pan, Z.; Lee, W.; Slutsky, L.; Clark, R.A.; Pernodet, N.; Rafailovich, M.H. Adverse effects of titanium dioxide nanoparticles on human dermal fibroblasts and how to protect cells. Small 2009, 5, 511–520. [Google Scholar] [CrossRef] [PubMed]
- von der Kammer, F.; Ottofuelling, S.; Hofmann, T. Assessment of the physico-chemical behavior of titanium dioxide nanoparticles in aquatic environments using multi-dimensional parameter testing. Environ. Pollut. 2010, 158, 3472–3481. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Marcellini, F.; Nepote, E.; Damiani, E.; Danovaro, R. Impact of inorganic UV filters contained in sunscreen products on tropical stony corals (Acropora spp.). Sci. Total Environ. 2018, 637, 1279–1285. [Google Scholar] [CrossRef]
- Brun, N.R.; Lenz, M.; Wehrli, B.; Fent, K. Comparative effects of zinc oxide nanoparticles and dissolved zinc on zebrafish embryos and eleuthero-embryos: Importance of zinc ions. Sci. Total Environ. 2014, 476, 657–666. [Google Scholar] [CrossRef]
- Schmidt, J.; Vogelsberger, W. Dissolution kinetics of titanium dioxide nanoparticles: The observation of an unusual kinetic size effect. J. Phys. Chem. B 2006, 110, 3955–3963. [Google Scholar] [CrossRef]
- Garoli, D.; Pelizzo, M.G.; Bernardini, B.; Nicolosi, P.; Alaibac, M. Sunscreen tests: Correspondence between in vitro data and values reported by the manufacturers. J. Dermatol. Sci. 2008, 52, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Uchino, T.; Tokunaga, H.; Ando, M.; Utsumi, H. Quantitative determination of OH radical generation and its cytotoxicity induced by TiO2–UVA treatment. Toxicology 2002, 16, 629–635. [Google Scholar] [CrossRef]
- Dunford, R.; Salinaro, A.; Cai, L.; Serpone, N.; Horikoshi, S.; Hidaka, H.; Knowland, J. Chemical oxidation and DNA damage catalysed by inorganic sunscreen ingredients. FEBS Lett. 1997, 418, 87–90. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, B.; Torres-Duarte, C.; Cherr, G.; Adams, N. Effects of three zinc-containing sunscreens on development of purple sea urchin (Strongylocentrotus purpuratus) embryos. Aquat. Toxicol. 2020, 218, 105355. [Google Scholar] [CrossRef]
- Peck, A.M. Analytical methods for the determination of persistent ingredients of personal care products in environmental matrices. Anal. Bioanal. Chem. 2006, 386, 907–939. [Google Scholar] [CrossRef]
- Valet, B.; Mayor, M.; Fitoussi, F.; Capellier, R.; Dormoy, M.; Ginestar, J. Colouring agents in cosmetic products (excluding hair dyes): Types of decorative cosmetic products. In Analysis of Cosmetic Products; Elsevier: Amsterdam, The Netherlands, 2007; pp. 141–152. [Google Scholar]
- Quartier, S.; Garmyn, M.; Becart, S.; Goossens, A. Allergic contact dermatitis to copolymers in cosmetics–case report and review of the literature. Contact Dermat. 2006, 55, 257–267. [Google Scholar] [CrossRef]
- Tønning, K.; Jacobsen, E.; Pedersen, E.; Strange, M.; Poulsen, P.B.; Møller, L.; Boyd, H.B. Survey and Health Assessment of the exposure of 2 year-olds to chemical substances in Consumer Products; Danish Ministry of the Environment, Environmental Protection Agency: Odense, Denmark, 2009; Chapter 5; p. 91. [Google Scholar]
- Vecchiato, M.; Gregoris, E.; Barbaro, E.; Barbante, C.; Piazza, R.; Gambaro, A. Fragrances in the seawater of Terra Nova Bay, Antarctica. Sci. Total Environ. 2017, 593, 375–379. [Google Scholar] [CrossRef] [Green Version]
- Kung, T.A.; Lee, S.H.; Yang, T.C.; Wang, W.H. Survey of selected personal care products in surface water of coral reefs in Kenting National Park, Taiwan. Sci. Total Environ. 2018, 635, 1302–1307. [Google Scholar] [CrossRef]
- Zhao, X.; Qiu, W.; Zheng, Y.; Xiong, J.; Gao, C.; Hu, S. Occurrence, distribution, bioaccumulation, and ecological risk of bisphenol analogues, parabens and their metabolites in the Pearl River Estuary, South China. Ecotoxicol. Environ. Saf. 2019, 180, 43–52. [Google Scholar] [CrossRef]
- Baek, S.; Joo, S.H.; Blackwelder, P.; Toborek, M. Effects of coating materials on antibacterial properties of industrial and sunscreen-derived titanium-dioxide nanoparticles on Escherichia coli. Chemosphere 2018, 208, 196–206. [Google Scholar] [CrossRef]
- Othman, S.H.; Abdul Rashid, S.; Mohd Ghazi, T.I.; Abdullah, N. Dispersion and stabilization of photocatalytic TiO2 nanoparticles in aqueous suspension for coatings applications. J. Nanomater. 2012, 2012, 718214. [Google Scholar] [CrossRef] [Green Version]
- Tejamaya, M.; Römer, I.; Merrifield, R.C.; Lead, J.R. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ. Sci. Technol. 2012, 46, 7011–7017. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Zhang, W.; Gao, H.; Li, Y.; Tong, X.; Li, K.; Zhu, X.; Wang, Y.; Chen, Y. Behavior and potential impacts of metal-based engineered nanoparticles in aquatic environments. Nanomaterials 2017, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Jafry, H.R.; Liga, M.V.; Li, Q.; Barron, A.R. Simple route to enhanced photocatalytic activity of P25 titanium dioxide nanoparticles by silica addition. Environ. Sci. Technol. 2010, 45, 1563–1568. [Google Scholar] [CrossRef]
- Dalai, S.; Pakrashi, S.; Kumar, R.S.; Chandrasekaran, N.; Mukherjee, A. A comparative cytotoxicity study of TiO2 nanoparticles under light and dark conditions at low exposure concentrations. Toxicol. Res. 2012, 1, 116–130. [Google Scholar] [CrossRef]
- Wang, J.; Fan, Y. Lung injury induced by TiO2 nanoparticles depends on their structural features: Size, shape, crystal phases, and surface coating. Int. J. Mol. Sci. 2014, 15, 22258–22278. [Google Scholar] [CrossRef]
- Planchon, M.; Ferrari, R.; Guyot, F.; Gélabert, A.; Menguy, N.; Chanéac, C.; Thill, A.; Benedetti, M.F.; Spalla, O. Interaction between Escherichia coli and TiO2 nanoparticles in natural and artificial waters. Colloids Surf. B Biointerfaces 2013, 102, 158–164. [Google Scholar] [CrossRef]
- Rosen, M.; Kunjappu, J. Adsorption of surface-active agents at interfaces: The electrical double layer. Surfactants Interfacial Phenom. 2004, 4, 98. [Google Scholar]
- Raza, G.; Amjad, M.; Kaur, I.; Wen, D. Stability and aggregation kinetics of titania nanomaterials under environmentally realistic conditions. Environ. Sci. Technol. 2016, 50, 8462–8472. [Google Scholar] [CrossRef]
- Waychunas, G.A.; Kim, C.S.; Banfield, J.F. Nanoparticulate iron oxide minerals in soils and sediments: Unique properties and contaminant scavenging mechanisms. J. Nanoparticle Res. 2005, 7, 409–433. [Google Scholar] [CrossRef]
- Borm, P.; Klaessig, F.C.; Landry, T.D.; Moudgil, B.; Pauluhn, J.; Thomas, K.; Trottier, R.; Wood, S. Research strategies for safety evaluation of nanomaterials, part V: Role of dissolution in biological fate and effects of nanoscale particles. Toxicol. Sci. 2006, 90, 23–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, S.; Mudunkotuwa, I.A.; Rupasinghe, T.; Grassian, V.H. Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: Influence of pH, ionic strength, size, and adsorption of humic acid. Langmuir 2011, 27, 6059–6068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, J.; Yang, K.; Liu, J.; Lin, D. Physicochemical transformation and algal toxicity of engineered nanoparticles in surface water samples. Environ. Pollut. 2016, 211, 132–140. [Google Scholar] [CrossRef]
- Gilbert, B.; Huang, F.; Zhang, H.; Waychunas, G.A.; Banfield, J.F. Nanoparticles: Strained and stiff. Science 2004, 305, 651–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairbairn, E.A.; Keller, A.A.; Mädler, L.; Zhou, D.; Pokhrel, S.; Cherr, G.N. Metal oxide nanomaterials in seawater: Linking physicochemical characteristics with biological response in sea urchin development. J. Hazard. Mater. 2011, 192, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Franklin, N.M.; Rogers, N.J.; Apte, S.C.; Batley, G.E.; Gadd, G.E.; Casey, P.S. Comparative toxicity of nanoparticulate ZnO, bulk ZnO, and ZnCl2 to a freshwater microalga (Pseudokirchneriella subcapitata): The importance of particle solubility. Environ. Sci. Technol. 2007, 41, 8484–8490. [Google Scholar] [CrossRef]
- Odzak, N.; Kistler, D.; Behra, R.; Sigg, L. Dissolution of metal and metal oxide nanoparticles in aqueous media. Environ. Pollut. 2014, 191, 132–138. [Google Scholar] [CrossRef]
- Misra, S.K.; Nuseibeh, S.; Dybowska, A.; Berhanu, D.; Tetley, T.D.; Valsami-Jones, E. Comparative study using spheres, rods and spindle-shaped nanoplatelets on dispersion stability, dissolution and toxicity of CuO nanomaterials. Nanotoxicology 2014, 8, 422–432. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Kang, B.; Hicks, B.; Chancellor Jr, T.F.; Chu, B.H.; Wang, H.-T.; Keselowsky, B.G.; Ren, F.; Lele, T.P. The control of cell adhesion and viability by zinc oxide nanorods. Biomaterials 2008, 29, 3743–3749. [Google Scholar] [CrossRef]
- Li, X.; Lenhart, J.J.; Walker, H.W. Aggregation kinetics and dissolution of coated silver nanoparticles. Langmuir 2012, 28, 1095–1104. [Google Scholar] [CrossRef]
- Gelabert, A.; Sivry, Y.; Ferrari, R.; Akrout, A.; Cordier, L.; Nowak, S.; Menguy, N.; Benedetti, M.F. Uncoated and coated ZnO nanoparticle life cycle in synthetic seawater. Environ. Toxicol. Chem. 2014, 33, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Lowry, G.V.; Gregory, K.B.; Apte, S.C.; Lead, J.R. Transformations of Nanomaterials in the Environment; ACS Publications: Washington, DC, USA, 2012. [Google Scholar]
- Odzak, N.; Kistler, D.; Sigg, L. Influence of daylight on the fate of silver and zinc oxide nanoparticles in natural aquatic environments. Environ. Pollut. 2017, 226, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fel, J.-P.; Lacherez, C.; Bensetra, A.; Mezzache, S.; Béraud, E.; Léonard, M.; Allemand, D.; Ferrier-Pagès, C. Photochemical response of the scleractinian coral Stylophora pistillata to some sunscreen ingredients. Coral Reefs 2019, 38, 109–122. [Google Scholar] [CrossRef] [Green Version]
- Rodil, R.; Moeder, M.; Altenburger, R.; Schmitt-Jansen, M. Photostability and phytotoxicity of selected sunscreen agents and their degradation mixtures in water. Anal. Bioanal. Chem. 2009, 395, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Paredes, E.; Perez, S.; Rodil, R.; Quintana, J.B.; Beiras, R. Ecotoxicological evaluation of four UV filters using marine organisms from different trophic levels Isochrysis galbana, Mytilus galloprovincialis, Paracentrotus lividus, and Siriella armata. Chemosphere 2014, 104, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Sureda, A.; Capo, X.; Busquets-Cortes, C.; Tejada, S. Acute exposure to sunscreen containing titanium induces an adaptive response and oxidative stress in Mytillus galloprovincialis. Ecotoxicol. Environ. Saf. 2018, 149, 58–63. [Google Scholar] [CrossRef]
- Araujo, C.V.M.; Rodriguez-Romero, A.; Fernandez, M.; Sparaventi, E.; Medina, M.M.; Tovar-Sanchez, A. Repellency and mortality effects of sunscreens on the shrimp Palaemon varians: Toxicity dependent on exposure method. Chemosphere 2020, 257, 127190. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Isobe, T.; Ramaswamy, B.R.; Chang, K.-H.; Amano, A.; Miller, T.M.; Siringan, F.P.; Tanabe, S. Contamination and bioaccumulation of benzotriazole ultraviolet stabilizers in fish from Manila Bay, the Philippines using an ultra-fast liquid chromatography-tandem mass spectrometry. Chemosphere 2011, 85, 751–758. [Google Scholar] [CrossRef]
- Nakata, H.; Murata, S.; Filatreau, J. Occurrence and concentrations of benzotriazole UV stabilizers in marine organisms and sediments from the Ariake Sea, Japan. Environ. Sci. Technol. 2009, 43, 6920–6926. [Google Scholar] [CrossRef]
- Fent, K.; Chew, G.; Li, J.; Gomez, E. Benzotriazole UV-stabilizers and benzotriazole: Antiandrogenic activity in vitro and activation of aryl hydrocarbon receptor pathway in zebrafish eleuthero-embryos. Sci. Total Environ. 2014, 482, 125–136. [Google Scholar] [CrossRef]
- Kim, J.-W.; Chang, K.-H.; Isobe, T.; Tanabe, S. Acute toxicity of benzotriazole ultraviolet stabilizers on freshwater crustacean (Daphnia pulex). J. Toxicol. Sci. 2011, 36, 247–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Romero, A.; Ruiz-Gutiérrez, G.; Viguri, J.R.; Tovar-Sánchez, A. Sunscreens as a New Source of Metals and Nutrients to Coastal Waters. Environ. Sci. Technol. 2019, 53, 10177–10187. [Google Scholar] [CrossRef] [PubMed]
- Zmozinski, A.V.; Pretto, T.; Borges, A.R.; Duarte, A.T.; Vale, M.G.R. Determination of Pb and Cr in sunscreen samples by high-resolution continuum source graphite furnace atomic absorption spectrometry and direct analysis. Microchem. J. 2016, 128, 89–94. [Google Scholar] [CrossRef]
- Zachariadis, G.; Sahanidou, E. Multi-element method for determination of trace elements in sunscreens by ICP-AES. J. Pharm. Biomed. Anal. 2009, 50, 342–348. [Google Scholar] [CrossRef]
- Labille, J.; Feng, J.; Botta, C.; Borschneck, D.; Sammut, M.; Cabie, M.; Auffan, M.; Rose, J.; Bottero, J.-Y. Aging of TiO2 nanocomposites used in sunscreen. Dispersion and fate of the degradation products in aqueous environment. Environ. Pollut. 2010, 158, 3482–3489. [Google Scholar] [CrossRef]
- Auffan, M.; Pedeutour, M.; Rose, J.; Masion, A.; Ziarelli, F.; Borschneck, D.; Chaneac, C.; Botta, C.; Chaurand, P.; Labille, J. Structural degradation at the surface of a TiO2-based nanomaterial used in cosmetics. Environ. Sci. Technol. 2010, 44, 2689–2694. [Google Scholar] [CrossRef]
- Chen, T.H.; Lin, C.C.; Meng, P.J. Zinc oxide nanoparticles alter hatching and larval locomotor activity in zebrafish (Danio rerio). J. Hazard. Mater. 2014, 277, 134–140. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, J.; Cai, Z. TiO2 nanoparticles in the marine environment: Impact on the toxicity of tributyltin to abalone (Haliotis diversicolor supertexta) embryos. Environ. Sci. Technol. 2011, 45, 3753–3758. [Google Scholar] [CrossRef]
- Sharma, V.; Anderson, D.; Dhawan, A. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria mediated apoptosis in human liver cells (HepG2). Apoptosis 2012, 17, 852–870. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, J.; Cai, Z. The toxicity and oxidative stress of TiO2 nanoparticles in marine abalone (Haliotis diversicolor supertexta). Mar. Pollut. Bull. 2011, 63, 334–338. [Google Scholar] [CrossRef]
- Krause, M.; Klit, A.; Blomberg Jensen, M.; Søeborg, T.; Frederiksen, H.; Schlumpf, M.; Lichtensteiger, W.; Skakkebaek, N.; Drzewiecki, K. Sunscreens: Are they beneficial for health? An overview of endocrine disrupting properties of UV-filters. Int. J. Androl. 2012, 35, 424–436. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, X.; Dzakpasu, M.; Wang, X.C. Evaluation of ecotoxicological effects of benzophenone UV filters: Luminescent bacteria toxicity, genotoxicity and hormonal activity. Ecotoxicol. Environ. Saf. 2017, 142, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, D.; Sieratowicz, A.; Zielke, H.; Oetken, M.; Hollert, H.; Oehlmann, J. Ecotoxicological effect characterisation of widely used organic UV filters. Environ. Pollut. 2012, 163, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Baker, T.J.; Tyler, C.R.; Galloway, T.S. Impacts of metal and metal oxide nanoparticles on marine organisms. Environ. Pollut. 2014, 186, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Spisni, E.; Seo, S.; Joo, S.H.; Su, C. Release and toxicity comparison between industrial- and sunscreen-derived nano-ZnO particles. Int. J. Environ. Sci. Technol. 2016, 13, 2485–2494. [Google Scholar] [CrossRef] [Green Version]
- Sendra, M.; Sanchez-Quiles, D.; Blasco, J.; Moreno-Garrido, I.; Lubian, L.M.; Perez-Garcia, S.; Tovar-Sanchez, A. Effects of TiO2 nanoparticles and sunscreens on coastal marine microalgae: Ultraviolet radiation is key variable for toxicity assessment. Environ. Int. 2017, 98, 62–68. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Damiani, E.; Marcellini, F.; Falugi, C.; Tiano, L.; Brugè, F.; Danovaro, R. Sunscreen products impair the early developmental stages of the sea urchin Paracentrotus lividus. Sci. Rep. 2017, 7, 7815. [Google Scholar] [CrossRef] [Green Version]
- Contier, S.M.D. Nanotechnology: Basic Calculations for Engineers and Scientists; Wiley-Interscience: Hoboken, NJ, USA, 2006; p. 52. [Google Scholar]
- Chaudhuri, R.K.; Majewski, G. Amphiphilic microfine titanium dioxide: Its properties and application in sunscreen formulations. DCI 1998, 162, 24–31. [Google Scholar]
- Hundrinke, K.; Simon, M. Ecotoxic effect of photocatalytic active nanoparticles (TiO2) on algae and daphnids. Environ. Sci. Pollut. Res. 2006, 13, 225–232. [Google Scholar] [CrossRef]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.-J.; Quigg, A.; Santschi, P.H.; Sigg, L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386. [Google Scholar] [CrossRef] [Green Version]
- Perron, H.; Domain, C.; Roques, J.; Drot, R.; Simoni, E.; Catalette, H. Optimisation of accurate rutile TiO2 (110),(100),(101) and (001) surface models from periodic DFT calculations. Theor. Chem. Acc. 2007, 117, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V. Nanoparticle aggregation: Challenges to understanding transport and reactivity in the environment. J. Environ. Qual. 2010, 39, 1909–1924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, S.; Pokhrel, S.; Xia, T.; Gilbert, B.; Ji, Z.; Schowalter, M.; Rosenauer, A.; Damoiseaux, R.; Bradley, K.A.; Maedler, L.; et al. Use of a Rapid Cytotoxicity Screening Approach To Engineer a Safer Zinc Oxide Nanoparticle through Iron Doping. Acs Nano 2010, 4, 15–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, W.; Mashayekhi, H.; Xing, B. Bacterial toxicity comparison between nano-and micro-scaled oxide particles. Environ. Pollut. 2009, 157, 1619–1625. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Logan, B.E. Bacterial adhesion to glass and metal-oxide surfaces. Colloids Surf. B Biointerfaces 2004, 36, 81–90. [Google Scholar] [CrossRef]
- Matranga, V.; Corsi, I. Toxic effects of engineered nanoparticles in the marine environment: Model organisms and molecular approaches. Mar. Environ. Res. 2012, 76, 32–40. [Google Scholar] [CrossRef]
- Peng, X.; Palma, S.; Fisher, N.S.; Wong, S.S. Effect of morphology of ZnO nanostructures on their toxicity to marine algae. Aquat. Toxicol. 2011, 102, 186–196. [Google Scholar] [CrossRef]
- Santos, A.J.M.; Miranda, M.S.; da Silva, J.C.E. The degradation products of UV filters in aqueous and chlorinated aqueous solutions. Water Res. 2012, 46, 3167–3176. [Google Scholar] [CrossRef]
- Manzo, S.; Schiavo, S.; Oliviero, M.; Toscano, A.; Ciaravolo, M.; Cirino, P. Immune and reproductive system impairment in adult sea urchin exposed to nanosized ZnO via food. Sci. Total Environ. 2017, 599–600, 9–13. [Google Scholar] [CrossRef]
- Osmond, M.J.; Mccall, M.J. Zinc oxide nanoparticles in modern sunscreens: An analysis of potential exposure and hazard. Nanotoxicology 2010, 4, 15–41. [Google Scholar] [CrossRef]
- Miller, R.J.; Lenihan, H.S.; Muller, E.B.; Tseng, N.; Hanna, S.K.; Keller, A.A. Impacts of metal oxide nanoparticles on marine phytoplankton. Environ. Sci. Technol. 2010, 44, 7329–7334. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xia, B.; Chen, B.; Sun, X.; Zhu, L.; Zhao, J.; Du, P.; Xing, B. Trophic transfer of TiO 2 nanoparticles from marine microalga (Nitzschia closterium) to scallop (Chlamys farreri) and related toxicity. Environ. Sci. Nano 2017, 4, 415–424. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Zhang, X.; Chang, Y.; Chen, Y. Trophic transfer of TiO2 nanoparticles from daphnia to zebrafish in a simplified freshwater food chain. Chemosphere 2010, 79, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Fent, K.; Kunz, P.Y.; Gomez, E. UV Filters in the Aquatic Environment Induce Hormonal Effects and Affect Fertility and Reproduction in Fish. Chim. Int. J. Chem. 2008, 62, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Gomez, E.; Bachelot, M.; Boillot, C.; Munaron, D.; Chiron, S.; Casellas, C.; Fenet, H. Bioconcentration of Two Pharmaceuticals (Benzodiazepines) and Two Personal Care Products (UV Filters) in Marine Mussels (Mytilus galloprovincialis) under Controlled Laboratory Conditions. Environ. Sci. Pollut. Res. 2012, 19, 2561–2569. [Google Scholar] [CrossRef]
- Gonzalez, L.; Lison, D.; Kirschvolders, M. Genotoxicity of engineered nanomaterials: A critical review. Nanotoxicology 2008, 2, 252–273. [Google Scholar] [CrossRef]
- Magdolenova, Z.; Collins, A.; Kumar, A.; Dhawan, A.; Stone, V.; Dusinska, M. Mechanisms of genotoxicity. A review of in vitro and in vivo studies with engineered nanoparticles. Nanotoxicology 2014, 8, 233–278. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, X.; Lao, Y.; Lv, X.; Tao, Y.; Huang, B.; Wang, J.; Zhou, J.; Cai, Z. TiO2 nanoparticles in the marine environment: Physical effects responsible for the toxicity on algae Phaeodactylum tricornutum. Sci. Total Environ. 2016, 565, 818–826. [Google Scholar] [CrossRef]
- Miao, A.-J.; Luo, Z.; Chen, C.-S.; Chin, W.-C.; Santschi, P.H.; Quigg, A. Intracellular uptake: A possible mechanism for silver engineered nanoparticle toxicity to a freshwater alga Ochromonas danica. PLoS ONE 2010, 5, e15196. [Google Scholar] [CrossRef] [Green Version]
- Schwegmann, H.; Feitz, A.J.; Frimmel, F.H. Influence of the zeta potential on the sorption and toxicity of iron oxide nanoparticles on S. cerevisiae and E. coli. J. Colloid Interface Sci. 2010, 347, 43–48. [Google Scholar] [CrossRef]
- Zhu, X.; Chang, Y.; Chen, Y. Toxicity and bioaccumulation of TiO2 nanoparticle aggregates in Daphnia magna. Chemosphere 2010, 78, 209–215. [Google Scholar] [CrossRef]
- Chen, J.; Dong, X.; Xin, Y.; Zhao, M. Effects of titanium dioxide nano-particles on growth and some histological parameters of zebrafish (Danio rerio) after a long-term exposure. Aquat. Toxicol. 2011, 101, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Doyle, J.J.; Ward, J.E.; Mason, R. Exposure of bivalve shellfish to titania nanoparticles under an environmental-spill scenario: Encounter, ingestion and egestion. J. Mar. Biol. Assoc. 2016, 96, 137–149. [Google Scholar] [CrossRef]
- Montes, M.O.; Hanna, S.K.; Lenihan, H.S.; Keller, A.A. Uptake, accumulation, and biotransformation of metal oxide nanoparticles by a marine suspension-feeder. J. Hazard. Mater. 2012, 225, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Yang, Y.; Tao, Y.; Jiang, Y.; Chen, B.; Zhu, X.; Cai, Z.; Li, B. A mechanism study on toxicity of graphene oxide to Daphnia magna: Direct link between bioaccumulation and oxidative stress. Environ. Pollut. 2018, 234, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Zhang, Y.; Song, C.; Zhu, X.; Xing, B. Bioaccumulation and biotransformation of polybrominated diphenyl ethers in the marine bivalve (Scapharca subcrenata): Influence of titanium dioxide nanoparticles. Mar. Pollut. Bull. 2015, 90, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Schilling, K.; Bradford, B.; Castelli, D.; Dufour, E.; Nash, J.F.; Pape, W.; Schulte, S.; Tooley, I.; van den Bosch, J.; Schellauf, F. Human safety review of “nano” titanium dioxide and zinc oxide. Photochem. Photobiol. Sci. 2010, 9, 495–509. [Google Scholar] [CrossRef] [PubMed]
- Senzui, M.; Tamura, T.; Miura, K.; Ikarashi, Y.; Watanabe, Y.; Fujii, M. Study on penetration of titanium dioxide (TiO2) nanoparticles into intact and damaged skin in vitro. J. Toxicol. Sci. 2010, 35, 107–113. [Google Scholar] [CrossRef] [Green Version]
- Dussert, A.S.; Gooris, E.; Hemmerle, J. Characterization of the mineral content of a physical sunscreen emulsion and its distribution onto human stratum corneum. Int. J. Cosmet. Sci. 1997, 19, 119–129. [Google Scholar] [CrossRef]
- Zvyagin, A.V.; Zhao, X.; Gierden, A.; Sanchez, W.; Ross, J.; Roberts, M.S. Imaging of zinc oxide nanoparticle penetration in human skin in vitro and in vivo. J. Biomed. Opt. 2008, 13, 064031. [Google Scholar] [CrossRef] [Green Version]
- Clemente, Z.; Castro, V.; Feitosa, L.; Lima, R.; Jonsson, C.; Maia, A.; Fraceto, L. Fish exposure to nano-TiO2 under different experimental conditions: Methodological aspects for nanoecotoxicology investigations. Sci. Total Environ. 2013, 463, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Clemente, Z.; Castro, V.; Moura, M.; Jonsson, C.; Fraceto, L. Toxicity assessment of TiO2 nanoparticles in zebrafish embryos under different exposure conditions. Aquat. Toxicol. 2014, 147, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-M.; An, Y.-J. Effects of zinc oxide and titanium dioxide nanoparticles on green algae under visible, UVA, and UVB irradiations: No evidence of enhanced algal toxicity under UV pre-irradiation. Chemosphere 2013, 91, 536–544. [Google Scholar] [CrossRef]
- Ji, J.; Long, Z.; Lin, D. Toxicity of oxide nanoparticles to the green algae Chlorella sp. Chem. Eng. J. 2011, 170, 525–530. [Google Scholar] [CrossRef]
- Hanson, K.M.; Gratton, E.; Bardeen, C.J. Sunscreen enhancement of UV-induced reactive oxygen species in the skin. Free. Radic. Biol. Med. 2006, 41, 1205–1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuzuki, T.; He, R.; Wang, J.; Sun, L.; Wang, X.; Hocking, R. Reduction of the photocatalytic activity of ZnO nanoparticles for UV protection applications. Int. J. Nanotechnol. 2012, 9, 1017–1029. [Google Scholar] [CrossRef]
- Livraghi, S.; Corazzari, I.; Paganini, M.C.; Ceccone, G.; Giamello, E.; Fubini, B.; Fenoglio, I. Decreasing the oxidative potential of TiO2 nanoparticles through modification of the surface with carbon: A new strategy for the production of safe UV filters. Chem. Commun. 2010, 46, 8478–8480. [Google Scholar] [CrossRef] [PubMed]
- Miao, A.J.; Zhang, X.Y.; Luo, Z.; Chen, C.S.; Chin, W.C.; Santschi, P.H.; Quigg, A. Zinc oxide–engineered nanoparticles: Dissolution and toxicity to marine phytoplankton. Environ. Toxicol. Chem. 2010, 29, 2814–2822. [Google Scholar] [CrossRef]
- Tang, C.H.; Lin, C.Y.; Lee, S.H.; Wang, W.H. Membrane lipid profiles of coral responded to zinc oxide nanoparticle-induced perturbations on the cellular membrane. Aquat. Toxicol. 2017, 187, 72–81. [Google Scholar] [CrossRef]
- Smijs, T.G.; Pavel, S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol. Sci. Appl. 2011, 4, 95. [Google Scholar] [CrossRef] [Green Version]
- Jeon, S.-k.; Kim, E.-j.; Lee, J.; Lee, S. Potential risks of TiO2 and ZnO nanoparticles released from sunscreens into outdoor swimming pools. J. Hazard. Mater. 2016, 317, 312–318. [Google Scholar] [CrossRef]
- Batley, G.E.; Kirby, J.K.; McLaughlin, M.J. Fate and risks of nanomaterials in aquatic and terrestrial environments. Acc. Chem. Res. 2012, 46, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Zhang, Y.; Song, C.; Zhu, X.; Xing, B. Titanium dioxide nanoparticles as carrier facilitate bioaccumulation of phenanthrene in marine bivalve, ark shell (Scapharca subcrenata). Environ. Pollut. 2014, 192, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Tian, S.; Lv, X.; Chen, Z.; Chen, B.; Zhu, X.; Cai, Z. TiO2 nanoparticles in the marine environment: Impact on the toxicity of phenanthrene and Cd2+ to marine zooplankton Artemia salina. Sci. Total Environ. 2018, 615, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhou, Q. Health and ecosystem risks of graphene. Chem. Rev. 2013, 113, 3815–3835. [Google Scholar] [CrossRef] [PubMed]


| Inorganic UVFs | Organism | Exposure Conditions | Effects | MoA | References |
|---|---|---|---|---|---|
| TiO2 (release from cosmetic products) | Algae (Thalassiosira pseudonana) | 0–96 h; 0.13–100 mg/L | Growth inhibition | Potential ROS production | [103] |
| nTiO2 from sunscreens | Chaetoceros gracilis (Bacillariophyceae); Amphidinium carterae (Dinophyceae); Pleurochrysis roscoffensis (Primnesiophycae); Nannochloropsis gaditana (Eustigmatophyceae) | 75 h; sunscreens (1–200 mg/L) or nTiO2 (1–10 mg/L) | Distribution of phytoplankton | H2O2 produced adsorption and absorption by the phytoplankton, membrane damage, ROS, and perhaps genotoxic damage | [104] |
| nTiO2 from sunscreen | Sea urchin (Paracentrotus lividus) | 3 h, 24 h; 10, 20, and 50 μL/L sunscreen | Sea urchin development impairment | decrease in AChE activity | [105] |
| nZnO (sunscreen-derived) | Algae (Thalassiosira pseudonana) | 0–96 h, 10 and 50 mg/L | Growth inhibition | Time- and concentration-dependent bioaccumulation | [106] |
| ZnO from sunscreen | Stony corals (Acropora spp.) | 48 h of in situ condition 6.3 mg/L | Coral bleaching; release of zooxanthellae | dissolved Zn2+ Zn2+ shading effects | [43] |
| zinc-containing sunscreens | Sea urchin (Strongylocentrotus purpuratus) embryos | 96 h; 0.01–1 mg/L | Malformations (skeletal abnormality, stage arrest, and axis determination disruption) | Zn2+ internalized | [49] |
| nTiO2 and nZnO from sunscreen | Shrimp (Palaemon varians) | 4 h 0–300 mg/L sunscreen | Repellency and mortality effects | [85] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, S.; Huang, J.; Jiang, X.; Huang, Y.; Zhu, X.; Cai, Z. Environmental Fate and Toxicity of Sunscreen-Derived Inorganic Ultraviolet Filters in Aquatic Environments: A Review. Nanomaterials 2022, 12, 699. https://doi.org/10.3390/nano12040699
Yuan S, Huang J, Jiang X, Huang Y, Zhu X, Cai Z. Environmental Fate and Toxicity of Sunscreen-Derived Inorganic Ultraviolet Filters in Aquatic Environments: A Review. Nanomaterials. 2022; 12(4):699. https://doi.org/10.3390/nano12040699
Chicago/Turabian StyleYuan, Shengwu, Jingying Huang, Xia Jiang, Yuxiong Huang, Xiaoshan Zhu, and Zhonghua Cai. 2022. "Environmental Fate and Toxicity of Sunscreen-Derived Inorganic Ultraviolet Filters in Aquatic Environments: A Review" Nanomaterials 12, no. 4: 699. https://doi.org/10.3390/nano12040699
APA StyleYuan, S., Huang, J., Jiang, X., Huang, Y., Zhu, X., & Cai, Z. (2022). Environmental Fate and Toxicity of Sunscreen-Derived Inorganic Ultraviolet Filters in Aquatic Environments: A Review. Nanomaterials, 12(4), 699. https://doi.org/10.3390/nano12040699