Radiosensitization Effect of Gold Nanoparticles on Plasmid DNA Damage Induced by Therapeutic MV X-rays
Abstract
:1. Introduction
2. Materials and Methods
2.1. AuNPs and DNA
2.2. Irradiation Conditions
2.3. DNA Damage Analysis
2.4. Measurement of ROS Yields
3. Results and Discussion
3.1. Characterization of AuNPs
3.2. Increase in DNA Damage by +AuNPs
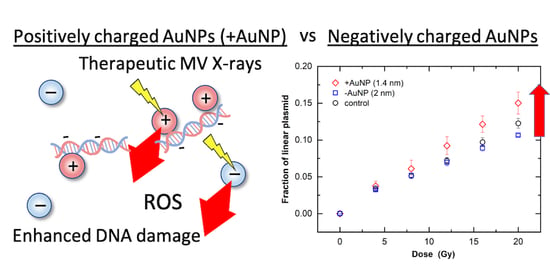
3.3. Effects of Surface Charge of AuNPs
3.4. Radiosensitization Mechanism for MV X-rays
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, F.M.; Gibbons, J.P. Khan’s The Physics of Radiation Therapy, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014; pp. 430–453. [Google Scholar]
- Ezzell, G.A.; Galvin, J.M.; Low, D.; Palta, J.R.; Rosen, I.; Sharpe, M.B.; Xia, P.; Xiao, Y.; Xing, L.; Yu, C.X. Guidance document on delivery, treatment planning, and clinical implementation of IMRT: Report of the IMRT Subcommittee of the AAPM Radiation Therapy Committee. Med. Phys. 2003, 30, 2089–2115. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Dilmanian, F.A.; Slatkin, D.N.; Smilowitz, H.M. Radiotherapy enhancement with gold nanoparticles. J. Pharm. Pharmacol. 2008, 60, 977–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Retif, P.; Pinel, S.; Toussaint, M.; Frochot, C.; Chouikrat, R.; Bastogne, T.; Barberi-Heyob, M. Nanoparticles for radiation therapy enhancement: The key parameters. Theranostics 2015, 5, 1030–1044. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yang, J.; Fu, S.; Wu, J. Gold nanoparticles as radiosensitizers in cancer radiotherapy. Int. J. Nanomed. 2020, 15, 9407–9430. [Google Scholar] [CrossRef] [PubMed]
- Misawa, M.; Takahashi, J. Generation of reactive oxygen species induced by gold nanoparticles under x-ray and UV irradiations. Nanomedicine 2011, 7, 604–614. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, N309–N315. [Google Scholar] [CrossRef]
- Butterworth, K.T.; Coulter, J.A.; Jain, S.; McMahon, S.J.; Schettino, G.; Prise, K.M.; Currell, F.J.; Hirst, D.G. Evaluation of cytotoxicity and radiation enhancement using 1.9 nm gold particles: Potential application for cancer therapy. Nanotechnology 2010, 23, 295101. [Google Scholar] [CrossRef] [Green Version]
- Hainfeld, J.F.; Smilowitz, H.M.; O’Connor, M.J.; Dilmanian, F.A.; Slatkin, D.N. Gold nanoparticle imaging and radiotherapy of brain tumors in mice. Nanomedicine 2013, 8, 1601–1609. [Google Scholar] [CrossRef] [Green Version]
- Lechtman, E.; Chattopadhyay, N.; Cai, Z.; Mashouf, S.; Reilly, R.; Pignol, J.P. Implications on clinical scenario of gold nanoparticle radiosensitization in regards to photon energy, nanoparticle size, concentration and location. Phys. Med. Biol. 2011, 56, 4631–4647. [Google Scholar] [CrossRef]
- Jain, S.; Coulter, J.A.; Hounsell, A.R.; Butterworth, K.T.; McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Dickson, G.R.; Prise, K.M.; Currell, F.J.; et al. Cell-specific radiosensitization by gold nanoparticles at megavoltage radiation energies. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 531–539. [Google Scholar] [CrossRef] [Green Version]
- Chithrani, D.B.; Jelveh, S.; Jalali, F.; Prooijen, M.; Allen, C.; Bristow, R.G.; Hill, R.P.; Jaffrayet, D.A. Gold nanoparticles as radiation sensitizers in cancer therapy. Radiat. Res. 2010, 173, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Chang-Hai, W.; Shin-Tai, C.; Chen, H.; Leng, W.; Chien, C.; Wang, C.; Kempson, I.M.; Hwu, Y.; Lai, T.; et al. Enhancement of cell radiation sensitivity by pegylated gold nanoparticles. Phys. Med. Biol. 2010, 55, 931–945. [Google Scholar] [CrossRef]
- Wolfe, T.; Chatterjee, D.; Lee, J.; Grant, J.D.; Bhattarai, S.; Tailor, R.; Goodrich, G.; Nicolucci, P.; Krishnan, S. Targeted gold nanoparticles enhance sensitization of prostate tumors to megavoltage radiation therapy in vivo. Nanomedicine 2015, 11, 1277–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Li, X.; Wang, Y.; Liu, Z.; Fu, L.; Hu, L. Enhancement of radiation effect and increase of apoptosis in lung cancer cells by thio-glucose-bound gold nanoparticles at megavoltage radiation energies. J. Nanopart. Res. 2013, 15, 1642. [Google Scholar] [CrossRef]
- Dou, Y.; Guo, Y.; Li, X.; Li, X.; Wang, S.; Wang, L.; Lv, G.; Zhang, X.; Wang, H.; Gong, X.; et al. Size-tuning ionization to optimize gold nanoparticles for simultaneous enhanced CT imaging and radiotherapy. ACS Nano 2016, 10, 2536–2548. [Google Scholar] [CrossRef]
- Butterworth, K.T.; Wyer, J.A.; Brennan-Fournet, M.; Latimer, C.J.; Shah, M.B.; Currell, F.J.; Hirst, D.G. Variation of strand break yield for plasmid DNA irradiated with high-Z metal nanoparticles. Radiat. Res. 2008, 170, 381–387. [Google Scholar] [CrossRef]
- McMahon, S.J.; Hyland, W.B.; Brun, E.; Butterworth, K.T.; Coulter, J.A.; Douki, T.; Hirst, D.G.; Jain, S.; Kavanagh, A.P.; Krpetic, Z.; et al. Dependence of gold nanoparticle radiosensitization in plasmid DNA. Phys. Chem. 2011, 115, 20160–20167. [Google Scholar] [CrossRef]
- Brun, E.; Sanche, L.; Sicard-Roselli, C. Parameters governing gold nanoparticle X-ray radiosensitization of DNA in solution. Colloids Surf. B Biointerfaces 2009, 72, 128–134. [Google Scholar] [CrossRef]
- Morozov, K.V.; Kolyvanova, M.A.; Kartseva, M.E.; Shishmakova, E.M.; Dement’eva, O.V.; Isagulieva, A.K.; Salpagarov, M.H.; Belousov, A.V.; Rudoy, V.M.; Shtil, A.A.; et al. Radiosensitization by gold nanoparticles: Impact of the size, dose rate, and photon energy. Nanomaterials 2020, 10, 952. [Google Scholar] [CrossRef]
- Foley, E.A.; Carter, J.D.; Shan, F.; Guo, T. Enhanced relaxation of nanoparticle-bound supercoiled DNA in X-ray radiation. Chem. Commun. 2005, 25, 3192–3194. [Google Scholar] [CrossRef]
- Yogo, K.; Misawa, M.; Shimizu, M.; Shimizu, H.; Kitagawa, T.; Hirayama, R.; Ishiyama, H.; Furukawa, T.; Yasuda, H. Effect of gold nanoparticle radiosensitization on plasmid DNA damage induced by high-dose rate brachytherapy. Int. J. Nanomed. 2021, 16, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Newton, G.L.; Ward, J.F.; Fahey, R.C. Aerobic radioprotection of pBR322 by thiols: Effect of thiol net charge upon scavenging of hydroxyl radicals and repair of DNA radicals. Radiat. Res. 1992, 130, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Yogo, K.; Murayama, C.; Fujisawa, Y.; Maeyama, T.; Hirayama, R.; Ogawa, Y.; Matsumoto, K.; Nakanishi, I.; Yasuda, H.; Ishiyama, H.; et al. Potential mechanisms for protective effect of D-methionine on plasmid DNA damage induced by therapeutic carbon ions. Radiat. Res. 2020, 193, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Yogo, K.; Murayama, C.; Hirayama, R.; Matsumoto, K.; Nakanishi, I.; Ishiyama, H.; Yasuda, H. Protective effect of amino acids on plasmid DNA damage induced by therapeutic carbon ions. Radiat. Res. 2021, 196, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Almond, P.R.; Biggs, P.J.; Coursey, B.M.; Hanson, W.F.; Huq, M.S.; Nath, R.; Rogers, D.W. AAPM’s TG-51 protocol for clinical reference dosimetry of high-energy photon and electron beams. Med. Phys. 1999, 26, 1847–1870. [Google Scholar] [CrossRef] [Green Version]
- Yogo, K.; Ogawa, T.; Hayashi, M.; Harada, Y.; Nishizaka, T.; Kinosita, K., Jr. Direct observation of strand passage by DNA-topoisomerase and its limited processivity. PLoS ONE 2012, 7, e34920. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, R.S.; Haidle, C.W.; Robberson, D.L. Bleomycin-specific fragmentation of double-stranded DNA. Biochemistry 1978, 17, 1890–1896. [Google Scholar] [CrossRef]
- Hanai, R.; Yazu, M.; Hieda, K. On the experimental distinction between SSBs and DSBs in circular DNA. Int. J. Radiat. Biol. 1998, 73, 475–479. [Google Scholar] [CrossRef]
- Sicard-Roselli, C.; Brun, E.; Gilles, M.; Baldacchino, G.; Kelsey, C.; McQuaid, H.; Polin, C.; Wardlow, N.; Currell, F. A new mechanism for hydroxyl radical production in irradiated nanoparticle solutions. Small 2014, 10, 3338–3346. [Google Scholar] [CrossRef] [Green Version]
- Bobyk, L.; Edouard, M.; Deman, P.; Vautrin, M.; Pernet-Gallay, K.; Delaroche, J.; Adam, J.; Estève, F.; Ravanat, J.; Elleaume, H. Photoactivation of gold nanoparticles for glioma treatment. Nanomedicine 2013, 9, 1089–1097. [Google Scholar] [CrossRef]
- Cho, E.C.; Xie, J.; Wurm, P.A.; Xia, Y. Understanding the role of surface charges in cellular adsorption versus internalization by selectively removing gold nanoparticles on the cell surface with a I2/KI etchant. Nano Lett. 2009, 9, 1080–1084. [Google Scholar] [CrossRef] [PubMed]








| Experimental Conditions | Yield (breaks per Da per Gy) * | Dose Enhancement Factor ** | ||
|---|---|---|---|---|
| Single-Strand Breaks | Double-Strand Breaks | Single-Strand Breaks | Double-Strand Breaks | |
| 1.4 nm +AuNP | (9.7 ± 1.3) × 10−8 | (2.7 ± 0.1) × 10−9 | 1.4 ± 0.2 | 1.2 ± 0.1 |
| 2 nm −AuNP | (6.4 ± 0.4) × 10−8 | (2.0 ± 0.1) × 10−9 | 0.9 ± 0.1 | 0.9 ± 0.1 |
| Control | (6.8 ± 0.7) × 10−8 | (2.2 ± 0.1) × 10−9 | 1.0 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yogo, K.; Misawa, M.; Shimizu, H.; Kitagawa, T.; Hirayama, R.; Ishiyama, H.; Yasuda, H.; Kametaka, S.; Takami, S. Radiosensitization Effect of Gold Nanoparticles on Plasmid DNA Damage Induced by Therapeutic MV X-rays. Nanomaterials 2022, 12, 771. https://doi.org/10.3390/nano12050771
Yogo K, Misawa M, Shimizu H, Kitagawa T, Hirayama R, Ishiyama H, Yasuda H, Kametaka S, Takami S. Radiosensitization Effect of Gold Nanoparticles on Plasmid DNA Damage Induced by Therapeutic MV X-rays. Nanomaterials. 2022; 12(5):771. https://doi.org/10.3390/nano12050771
Chicago/Turabian StyleYogo, Katsunori, Masaki Misawa, Hidetoshi Shimizu, Tomoki Kitagawa, Ryoichi Hirayama, Hiromichi Ishiyama, Hiroshi Yasuda, Satoshi Kametaka, and Seiichi Takami. 2022. "Radiosensitization Effect of Gold Nanoparticles on Plasmid DNA Damage Induced by Therapeutic MV X-rays" Nanomaterials 12, no. 5: 771. https://doi.org/10.3390/nano12050771
APA StyleYogo, K., Misawa, M., Shimizu, H., Kitagawa, T., Hirayama, R., Ishiyama, H., Yasuda, H., Kametaka, S., & Takami, S. (2022). Radiosensitization Effect of Gold Nanoparticles on Plasmid DNA Damage Induced by Therapeutic MV X-rays. Nanomaterials, 12(5), 771. https://doi.org/10.3390/nano12050771