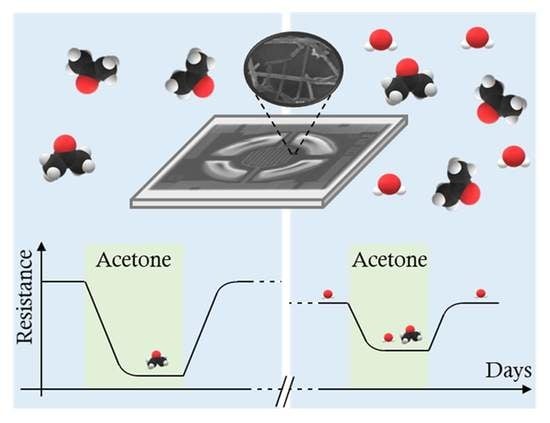
Role of Working Temperature and Humidity in Acetone Detection by SnO2 Covered ZnO Nanowire Network Based Sensors
Abstract
:1. Introduction
2. Gas Sensing Mechanisms
3. Materials and Methods
3.1. Device Fabrication
3.2. Gas Exposure
4. Results and Discussion
4.1. Device Aging
4.2. Sensor Response Stability
4.3. Water-Oxygen Interplay at Tin Oxide Surface
4.4. Effect of Encapsulating Layer
4.5. Sensor Characterization
4.5.1. Acetone Sensitivity
4.5.2. Sensor Selectivity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Statistics Relative to the Diameter of ZnO NWs in Nanonet

Appendix B. Sensor Response Linearity

Appendix C. Sensor Response Stability

Appendix D. Sensor Selectivity

References
- Anderson, J.C. Measuring Breath Acetone for Monitoring Fat Loss: Review. Obesity 2015, 23, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Y.; Lu, X.; Huang, Y.; Liu, Y.; Cheng, S.; Duan, Y. A Cross-Sectional Study of Breath Acetone Based on Diabetic Metabolic Disorders. J. Breath Res. 2015, 9, 016005. [Google Scholar] [CrossRef] [PubMed]
- Amann, A.; Miekisch, W.; Schubert, J.; Buszewski, B.; Ligor, T.; Jezierski, T.; Pleil, J.; Risby, T. Analysis of Exhaled Breath for Disease Detection. Annu. Rev. Anal. Chem. 2014, 7, 455–482. [Google Scholar] [CrossRef] [PubMed]
- Righettoni, M.; Tricoli, A.; Gass, S.; Schmid, A.; Amann, A.; Pratsinis, S.E. Analytica Chimica Acta Breath Acetone Monitoring by Portable Si:WO3 Gas Sensors. Anal. Chim. Acta 2012, 738, 69–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Natale, C.; Paolesse, R.; Martinelli, E.; Capuano, R. Solid-State Gas Sensors for Breath Analysis: A Review. Anal. Chim. Acta 2014, 824, 1–17. [Google Scholar] [CrossRef]
- Ferrus, L.; Guenard, H.; Vardon, G.; Varene, P. Respiratory Water Loss. Respir. Physiol. 1980, 39, 367–381. [Google Scholar] [CrossRef]
- Becker, T.; Ahlers, S.; Bosch-v.Braunmühl, C.; Müller, G.; Kiesewetter, O. Gas Sensing Properties of Thin- and Thick-Film Tin-Oxide Materials. Sens. Actuators B Chem. 2001, 77, 55–61. [Google Scholar] [CrossRef]
- Wang, L.; Teleki, A.; Pratsinis, S.E.; Gouma, P.I. Ferroelectric WO3 Nanoparticles for Acetone Selective Detection. Chem. Mater. 2008, 20, 4794–4796. [Google Scholar] [CrossRef]
- Sun, Y.-F.; Liu, S.-B.; Meng, F.-L.; Liu, J.-Y.; Jin, Z.; Kong, L.-T.; Liu, J.-H. Metal Oxide Nanostructures and Their Gas Sensing Properties: A Review. Sensors 2012, 12, 2610–2631. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Zeng, W.; Li, Y. Gas Sensing Mechanisms of Metal Oxide Semiconductors: A Focus Review. Nanoscale 2019, 11, 22664–22684. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, G.; Nagashima, K.; Takahashi, T.; Hosomi, T.; Yanagida, T. Metal–Oxide Nanowire Molecular Sensors and Their Promises. Chemosensors 2021, 9, 41. [Google Scholar] [CrossRef]
- Woo, H.-S.; Na, C.W.; Lee, J.-H. Design of Highly Selective Gas Sensors via Physicochemical Modification of Oxide Nanowires: Overview. Sensors 2016, 16, 1531. [Google Scholar] [CrossRef] [PubMed]
- Fierro, J.L.G. Metal Oxides: Chemistry and Applications; CRC Press: Boca Raton, FL, USA, 2005; ISBN 978-1-4200-2812-6. [Google Scholar]
- Mohammad, A.; Ehtisham Khan, M.; Alarifi, I.M.; Hwan Cho, M.; Yoon, T. A Sensitive Electrochemical Detection of Hydrazine Based on SnO2/CeO2 Nanostructured Oxide. Microchem. J. 2021, 171, 106784. [Google Scholar] [CrossRef]
- Chakraborty, S.; Banerjee, D.; Ray, I.; Sen, A. Detection of Biomarker in Breath: A Step towards Noninvasive Diabetes Monitoring. Curr. Sci. 2008, 94, 237–242. [Google Scholar]
- Wang, L.; Kalyanasundaram, K.; Stanacevic, M.; Gouma, P. Nanosensor Device for Breath Acetone Detection. Sen. Lett. 2010, 8, 709–712. [Google Scholar] [CrossRef]
- Righettoni, M.; Tricoli, A.; Pratsinis, S.E. Thermally Stable, Silica-Doped ε-WO3 for Sensing of Acetone in the Human Breath. Chem. Mater. 2010, 22, 3152–3157. [Google Scholar] [CrossRef]
- Wang, L.; Gouma, P. Selective Crystal Structure Synthesis and Sensing Dependencies. In Metal Oxide Nanomaterials for Chemical Sensors; Carpenter, M.A., Mathur, S., Kolmakov, A., Eds.; Springer: New York, NY, USA, 2013; pp. 167–188. ISBN 978-1-4614-5394-9. [Google Scholar]
- Santra, S.; De Luca, A.; Bhaumik, S.; Ali, S.Z.; Udrea, F.; Gardner, J.W.; Ray, S.K.; Guha, P.K. Dip pen nanolithography-deposited zinc oxide nanorods on a CMOS MEMS platform for ethanol sensing. RSC Adv. 2015, 5, 47609–47616. [Google Scholar] [CrossRef] [Green Version]
- Santra, S.; Sinha, A.K.; De Luca, A.; Ali, S.Z.; Udrea, F.; Guha, P.K.; Ray, S.K.; Gardner, J.W. Mask-less deposition of Au–SnO2 nanocomposites on CMOS MEMS platform for ethanol detection. Nanotechnology 2016, 27, 125502. [Google Scholar] [CrossRef]
- Morisot, F.; Zuliani, C.; Luque, J.; Ali, Z.; Mouis, M.; Nguyen, V.H.; Muñoz-Rojas, D.; Lourhzal, O.; Texier, M.; Cornelius, T.W.; et al. ZnO Based Nanowire Network for Gas Sensing Applications. Mater. Res. Express 2019, 6, 084004. [Google Scholar] [CrossRef]
- Barsan, N.; Schweizer-Berberich, M.; Göpel, W. Fundamental and Practical Aspects in the Design of Nanoscaled SnO2 Gas Sensors: A Status Report. Fresenius J. Anal. Chem. 1999, 365, 287–304. [Google Scholar] [CrossRef]
- Gurlo, A. Interplay between O2 and SnO2: Oxygen ionosorption and spectroscopic evidence for adsorbed oxygen. ChemPhysChem 2006, 7, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Yamazoe, N.; Suematsu, K.; Shimanoe, K. Extension of Receptor Function Theory to Include Two Types of Adsorbed Oxygen for Oxide Semiconductor Gas Sensors. Sens. Actuators B Chem. 2012, 163, 128–135. [Google Scholar] [CrossRef]
- Suematsu, K.; Ma, N.; Watanabe, K.; Yuasa, M.; Kida, T.; Shimanoe, K. Effect of Humid Aging on the Oxygen Adsorption in SnO2 Gas Sensors. Sensors 2018, 18, 254. [Google Scholar] [CrossRef] [Green Version]
- Grossmann, K.; Pavelko, R.G.; Barsan, N.; Weimar, U. Interplay of H2, Water Vapor and Oxygenat the Surface of SnO2 Based Gas Sensors—An Operando Investigation Utilizing Deuterated Gases. Sens. Actuators B Chem. 2012, 166–167, 787–793. [Google Scholar] [CrossRef]
- Ma, N.; Suematsu, K.; Yuasa, M.; Kida, T.; Shimanoe, K. Effect of Water Vapor on Pd-Loaded SnO2 Nanoparticles Gas Sensor. ACS Appl. Mater. Interfaces 2015, 7, 5863–5869. [Google Scholar] [CrossRef]
- Singh, M.; Kaur, N.; Drera, G.; Casotto, A.; Sangaletti, L.; Comini, E. SAM Functionalized ZnO Nanowires for Selective Acetone Detection: Optimized Surface Specific Interaction Using APTMS and GLYMO Monolayers. Adv. Funct. Mater. 2020, 30, 2003217. [Google Scholar] [CrossRef]
- Santra, S.; Guha, P.K.; Ali, S.Z.; Hiralal, P.; Unalan, H.E.; Covington, J.A.; Amaratunga, G.A.J.; Milne, W.I.; Gardner, J.W.; Udrea, F. ZnO Nanowires Grown on SOI CMOS Substrate for Ethanol Sensing. Sens. Actuators B Chem. 2010, 146, 559–565. [Google Scholar] [CrossRef]
- Clifford, P.K.; Tuma, D.T. Characteristics of Semiconductor Gas Sensors I. Steady State Gas Response. Sens. Actuators 1982, 3, 233–254. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Explicit Formulation for the Response of Neat Oxide Semiconductor Gas Sensor to Reducing Gas. Sens. Actuators B Chem. 2011, 158, 28–34. [Google Scholar] [CrossRef]
- Morisot, F.; Nguyen, V.H.; Montemont, C.; Maindron, T.; Muñoz-Rojas, D.; Mouis, M.; Langlet, M.; Ternon, C. Al2O3, Al Doped ZnO and SnO2 Encapsulation of Randomly Oriented ZnO Nanowire Networks for High Performance and Stable Electrical Devices. Nanotechnology 2019, 30, 385202. [Google Scholar] [CrossRef]
- Demes, T.; Ternon, C.; Morisot, F.; Riassetto, D.; Legallais, M.; Roussel, H.; Langlet, M. Mechanisms Involved in the Hydrothermal Growth of Ultra-Thin and High Aspect Ratio ZnO Nanowires. Appl. Surf. Sci. 2017, 410, 423–431. [Google Scholar] [CrossRef]
- Sysoev, V.V.; Schneider, T.; Goschnick, J.; Kiselev, I.; Habicht, W.; Hahn, H.; Strelcov, E.; Kolmakov, A. Percolating SnO2 Nanowire Network as a Stable Gas Sensor: Direct Comparison of Long-Term Performance versus SnO2 Nanoparticle Films. Sens. Actuators B Chem. 2009, 139, 699–703. [Google Scholar] [CrossRef]
- Ponzoni, A. Morphological Effects in SnO2 Chemiresistors for Ethanol Detection: A Review in Terms of Central Performances and Outliers. Sensors 2021, 21, 29. [Google Scholar] [CrossRef] [PubMed]
- Rydosz, A. Sensors for Enhanced Detection of Acetone as a Potential Tool for Noninvasive Diabetes Monitoring. Sensors 2018, 18, 2298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Q.; Cui, Y.; Zhu, L.; Yao, Y. Increasing Oxygen Vacancies at Room Temperature in SnO2 for Enhancing Ethanol Gas Sensing. Mater. Sci. Semicond. Processing 2020, 111, 104962. [Google Scholar] [CrossRef]
- Kao, K.W.; Hsu, M.C.; Chang, Y.H.; Gwo, S.; Andrew Yeh, J. A Sub-Ppm Acetone Gas Sensor for Diabetes Detection Using 10 nm Thick Ultrathin InN FETs. Sensors 2012, 12, 7157–7168. [Google Scholar] [CrossRef] [Green Version]
- Khuspe, G.D.; Sakhare, R.D.; Navale, S.T.; Chougule, M.A.; Kolekar, Y.D.; Mulik, R.N.; Pawar, R.C.; Lee, C.S.; Patil, V.B. Nanostructured SnO2 Thin Films for NO2 Gas Sensing Applications. Ceram. Int. 2013, 39, 8673–8679. [Google Scholar] [CrossRef]
- Kumar, R.; Al-Dossary, O.; Kumar, G.; Umar, A. Zinc Oxide Nanostructures for NO2 Gas–Sensor Applications: A Review. Nano-Micro Lett. 2014, 7, 97–120. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh, N.; Jamalabadi, H.; Tavoli, F. Breath Acetone Sensors as Non-Invasive Health Monitoring Systems: A Review. IEEE Sens. J. 2020, 20, 5–31. [Google Scholar] [CrossRef]
- Usman, F.; Dennis, J.O.; Ahmed, A.Y.; Meriaudeau, F.; Ayodele, O.B.; Rabih, A.A.S. A Review of Biosensors for Non-Invasive Diabetes Monitoring and Screening in Human Exhaled Breath. IEEE Access 2019, 7, 5963–5974. [Google Scholar] [CrossRef]
- Sysoev, V.V.; Kiselev, I.; Trouillet, V.; Bruns, M. Enhancing the Gas Selectivity of Single-Crystal SnO2: Pt Thin-Film Chemiresistor Microarray by SiO2 Membrane Coating. Sens. Actuators B Chem. 2013, 185, 59–69. [Google Scholar] [CrossRef]







| (2) and (3) Parameters | Measurement @ 350 °C in Dry Air | Measurement @ 350 °C in Wet Air (PH2O = 0.012 atm) | For a Given Ageing Atmosphere, Impact of Water on Surface Species: | |||
|---|---|---|---|---|---|---|
| K2dry | K3dry | K2wet | K3wet | |||
| Aging @ 580 °C | Dry Atmosphere During Aging | 8 × 10−11 | 8 × 10−41 | 1 × 10−11 | 3 × 10−49 | In Presence of Wet Atmosphere: K2 ↘: Density of O− ↘, K3 ↘↘: O2− blocked |
| Wet Atmosphere During Aging | 5 × 10−7 | 3 × 10−36 | 4 × 10−11 | 7 × 10−50 | ||
| For A Given Measurement Atmosphere, Impact of Water in the Ageing Atmosphere on Surface Species: | When aging is done in wet atmosphere: K2wet/dry and K3dry↗: equilibria are shifted to the right | |||||
| ↗↗ ↗↗ ) | ↗, more blocked | |||||
| Reducing Gas Acetone/Ethanol | Oxidizing Gas NO2 | |
|---|---|---|
| Sensor Response (S) |
| Bare | DLow | ILow | |
|---|---|---|---|
| Encapsulation by ALD | none | doped SnO2 | undoped SnO2 |
| Oxidizing Agent | - | H2O2 | H2O |
| Reducing Agent | - | TDMA(VI)Sn | TDMA(VI)Sn |
| Layer Thickness (nm) | - | 6 | 6 |
| Layer Conductivity (Ω−1 cm−1) at Room Temperature | - | 0.1 | 0 |
| Nanowire Density (NW cm−2) | 5 × 108 | 5 × 108 | 5 × 108 |
| Number of Functional Devices/Tested Devices (Humidity Study) | 4/4 | 4/4 | 3/4 |
| Number of Functional Devices/Tested Devices (Temperature Study) | NA | 4/4 | 4/4 |
| Humidity Study | Temperature Study | ||||
|---|---|---|---|---|---|
| Name | Dry/Humid | 230 | 300 | 370 | |
| Number of cycles | 17 | 3 | 3 | 9 | |
| Sub-cycle | Dry | Humid | - | - | - |
| Humidity (% RH) | 0 | 50 | 50 | ||
| Temperature (°C) | 370 | 230 | 300 | 370 | |
| Number of Gas Injection in One Cycle (Each Injection is Separated by a Purge) | 5 | 5 | 7 | ||
| Stabilization Time in Cycles Before 1st Acetone Injection (min) | 60 | 60 | |||
| Humidity Study | Temperature Study | ||||
|---|---|---|---|---|---|
| Sub Cycle | Dry | Humid | For Each Temperature | ||
| Gas Sequence | Acetone | Acetone | Acetone | Ethanol | NO2 |
| Concentration (ppm) | 2 | 2 | 0.5/1/2/5/10 | 5 | 0.1 |
| Number of Injection (Each One is Separated by a Purge) | 5 | 5 | 5 (one at each concentration) | 1 | 1 |
| Exposure Time/Purge Time (min) | 15/15 | 15/15 | 15/15 | 15/30 | 15/30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morisot, F.; Zuliani, C.; Mouis, M.; Luque, J.; Montemont, C.; Maindron, T.; Ternon, C. Role of Working Temperature and Humidity in Acetone Detection by SnO2 Covered ZnO Nanowire Network Based Sensors. Nanomaterials 2022, 12, 935. https://doi.org/10.3390/nano12060935
Morisot F, Zuliani C, Mouis M, Luque J, Montemont C, Maindron T, Ternon C. Role of Working Temperature and Humidity in Acetone Detection by SnO2 Covered ZnO Nanowire Network Based Sensors. Nanomaterials. 2022; 12(6):935. https://doi.org/10.3390/nano12060935
Chicago/Turabian StyleMorisot, Fanny, Claudio Zuliani, Mireille Mouis, Joaquim Luque, Cindy Montemont, Tony Maindron, and Céline Ternon. 2022. "Role of Working Temperature and Humidity in Acetone Detection by SnO2 Covered ZnO Nanowire Network Based Sensors" Nanomaterials 12, no. 6: 935. https://doi.org/10.3390/nano12060935
APA StyleMorisot, F., Zuliani, C., Mouis, M., Luque, J., Montemont, C., Maindron, T., & Ternon, C. (2022). Role of Working Temperature and Humidity in Acetone Detection by SnO2 Covered ZnO Nanowire Network Based Sensors. Nanomaterials, 12(6), 935. https://doi.org/10.3390/nano12060935