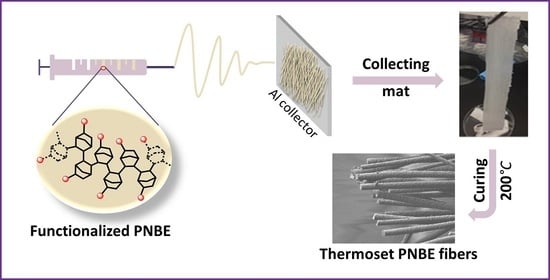
Ultra-High Tg Thermoset Fibers Obtained by Electrospinning of Functional Polynorbornenes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterizations
2.2. Co-Electrospinning of Polymer 1 with IPDA Cross-Linker (Typical Experiment)
2.3. Electrospinning of Polymer 2 (Typical Experiment)
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Ang, B.C.; Andriyana, A.; Afifi, A.M. A review on fabrication of nanofibers via electrospinning and their applications. SN Appl. Sci. 2019, 1, 1248. [Google Scholar] [CrossRef] [Green Version]
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial upscaling of electrospinning and applications of polymer nanofibers: A review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- Luo, C.J.; Stoyanov, S.D.; Stride, E.; Pelan, E.; Edirisinghe, M. Electrospinning versus fibre production methods: From specifics to technological convergence. Chem. Soc. Rev. 2012, 41, 4708. [Google Scholar] [CrossRef] [PubMed]
- Richard-Lacroix, M.; Pellerin, C. Molecular orientation in electrospun fibers: From mats to single fibers. Macromolecules 2013, 46, 9473–9493. [Google Scholar] [CrossRef]
- Chen, S.; Li, R.; Li, X.; Xie, J. Electrospinning: An enabling nanotechnology platform for drug delivery and regenerative medicine. Adv. Drug Deliv. Rev. 2018, 132, 188–213. [Google Scholar] [CrossRef]
- Jin, H.; Capareda, S.; Chang, Z.; Gao, J.; Xu, Y.; Zhang, J. Biochar pyrolytically produced from municipal solid wastes for aqueous As(V) removal: Adsorption property and its improvement with KOH activation. Bioresour. Technol. 2014, 169, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Kalaoglu-Altan, O.I.; Verbraeken, B.; Lava, K.; Gevrek, T.N.; Sanyal, R.; Dargaville, T.; De Clerck, K.; Hoogenboom, R.; Sanyal, A. Multireactive poly(2-oxazoline) nanofibers through electrospinning with crosslinking on the fly. ACS Macro Lett. 2016, 5, 676–681. [Google Scholar] [CrossRef]
- Wang, X.; Pellerin, C.; Bazuin, C.G. Enhancing the electrospinnability of low molecular weight polymers using small effective cross-linkers. Macromolecules 2016, 49, 891–899. [Google Scholar] [CrossRef]
- Huang, C.; Wang, S.; Zhang, H.; Li, T.; Chen, S.; Lai, C.; Hou, H. High strength electrospun polymer nanofibers made from BPDA–PDA polyimide. Eur. Polym. J. 2006, 42, 1099–1104. [Google Scholar] [CrossRef]
- Huang, C.; Chen, S.; Reneker, D.H.; Lai, C.; Hou, H. High-strength mats from electrospun poly(p-phenylene biphenyltetracarboximide) nanofibers. Adv. Mater. 2006, 18, 668–671. [Google Scholar] [CrossRef]
- Srinivasan, G.; Reneker, D.H. Structure and morphology of small diameter electrospun aramid fibers. Polym. Int. 1995, 36, 195–201. [Google Scholar] [CrossRef]
- Yao, L.; Lee, C.; Kim, J. Fabrication of electrospun meta-aramid nanofibers in different solvent systems. Fibers Polym. 2010, 11, 1032–1040. [Google Scholar] [CrossRef]
- Kim, J.; Reneker, D.H. Mechanical properties of composites using ultrafine electrospun fibers. Polym. Compos. 1999, 20, 124–131. [Google Scholar] [CrossRef]
- Kim, J.; Reneker, D.H. Polybenzimidazole nanofiber produced by electrospinning. Polym. Eng. Sci. 1999, 39, 849–854. [Google Scholar] [CrossRef]
- Lee, W.-J.; Kim, C.; Yang, K.-S.; Kim, J.-S.; Kim, S.-J. Supercapacitors prepared from carbon nanofibers electrospun from polybenzimidazol. J. Electrochem. Soc. 2004, 151, A769. [Google Scholar] [CrossRef]
- Duan, G.; Jiang, S.; Chen, S.; Hou, H. Heat and solvent resistant electrospun polybenzoxazole nanofibers from methoxy-containing polyaramide. J. Nanomater. 2010, 2010, 219562. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, S.; Duan, G.; Li, J.; Liu, K.; Zhou, C.; Hou, H. Heat-resistant polybenzoxazole nanofibers made by electrospinning. Eur. Polym. J. 2014, 50, 61–68. [Google Scholar] [CrossRef]
- Commarieu, B.; Potier, J.; Compaore, M.; Dessureault, S.; Goodall, B.L.; Li, X.; Claverie, J.P. Ultrahigh Tg epoxy thermosets based on insertion polynorbornenes. Macromolecules 2016, 49, 920–925. [Google Scholar] [CrossRef]
- Commarieu, B.; Potier, J.; Compaore, M.; de Boever, R.; Imbeault, R.; Claverie, J.P. A simple and efficient protocol for the catalytic insertion polymerization of functional norbornenes. J. Vis. Exp. 2017, 120, e54552. [Google Scholar] [CrossRef] [PubMed]
- Commarieu, B.; Claverie, J.P. Bypassing the lack of reactivity of endo-substituted norbornenes with the catalytic rectification–insertion mechanism. Chem. Sci. 2015, 6, 2172–2181. [Google Scholar] [CrossRef] [Green Version]
- Potier, J.; Commarieu, B.; Soldera, A.; Claverie, J.P. Thermodynamic control in the catalytic insertion polymerization of norbornenes as rationale for the lack of reactivity of endo-substituted norbornenes. ACS Catal. 2018, 8, 6047–6054. [Google Scholar] [CrossRef]
- Nie, X.; Wu, S.; Mensah, A.; Lu, K.; Wei, Q. Carbon quantum dots embedded electrospun nanofibers for efficient antibacterial photodynamic inactivation. Mater. Sci. Eng. C 2020, 108, 110377. [Google Scholar] [CrossRef] [PubMed]
- Baji, A.; Agarwal, K.; Oopath, S.V. Emerging developments in the use of electrospun fibers and membranes for protective clothing applications. Polymers 2020, 12, 492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serbezeanu, D.; Popa, A.M.; Stelzig, T.; Sava, I.; Rossi, R.M.; Fortunato, G. Preparation and characterization of thermally stable polyimide membranes by electrospinning for protective clothing applications. Text. Res. J. 2015, 85, 1763–1775. [Google Scholar] [CrossRef]
- de Boëver, R.; Langlois, A.; Li, X.; Claverie, J.P. Graphitic dots combining photophysical characteristics of organic molecular fluorophores and inorganic quantum dots. JACS Au 2021, 1, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Abbasi, F.; Claverie, J. An efficient templating approach for the synthesis of redispersible size-controllable carbon quantum dots from graphitic polymeric micelles. Chem.-Eur. J. 2015, 21, 15142–15147. [Google Scholar] [CrossRef] [PubMed]
- Steyaert, I.; Vancoillie, G.; Hoogenboom, R.; De Clerck, K. Dye immobilization in halochromic nanofibers through blend electrospinning of a dye-containing copolymer and polyamide-6. Polym. Chem. 2015, 6, 2685–2694. [Google Scholar] [CrossRef]





| Expt | Polymer | Cross-linker 1 | Mass Ratio 2 | Solvent | Injection 3 (mL/min) | Diameter 4 (µm) |
|---|---|---|---|---|---|---|
| 1 | 1 | IPDA | 83:17 | DMF/THF | 0.1 | 8.5 |
| 2 | 1 | IPDA | 75:25 | DMF/THF | 0.1 | 5.7 |
| 3 | 1 | IPDA | 67:33 | DMF/THF | 0.1 | 4.6 |
| 4 | 1 | IPDA | 50:50 | DMF/THF | 0.1 | 2.2 |
| 5 | 1 | IPDA | 67:33 | DMF/THF | 0.05 | 1.0 |
| 6 | 1 | IPDA | 67:33 | DMF/THF | 0.03 | 0.4 |
| 7 | 2 | DEA | 67:33 | Water | 0.5 | 1.6 |
| 8 | 2 | DEA | 85:15 | DMF | 0.002 | 0.3 |
| 9 | 2 | Glycerol | 67:33 | Ethanol | 0.5 | 2.9 |
| 10 | 2 | DGE | 60:40 | Ethanol | 0.01 | 0.5 |
| 11 | 2 | DGE | 60:40 | Ethanol | 0.03 | 0.6 |
| 12 | 2 | DGE | 60:40 | Ethanol | 0.05 | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Commarieu, B.; Compaoré, M.; de Boëver, R.; Imbeault, R.; Leprince, M.; Martin, B.; Perard, B.; Qiu, W.; Claverie, J.P. Ultra-High Tg Thermoset Fibers Obtained by Electrospinning of Functional Polynorbornenes. Nanomaterials 2022, 12, 967. https://doi.org/10.3390/nano12060967
Commarieu B, Compaoré M, de Boëver R, Imbeault R, Leprince M, Martin B, Perard B, Qiu W, Claverie JP. Ultra-High Tg Thermoset Fibers Obtained by Electrospinning of Functional Polynorbornenes. Nanomaterials. 2022; 12(6):967. https://doi.org/10.3390/nano12060967
Chicago/Turabian StyleCommarieu, Basile, Moubarak Compaoré, Raphaël de Boëver, Régis Imbeault, Maxime Leprince, Barbara Martin, Bruno Perard, Weiguang Qiu, and Jerome P. Claverie. 2022. "Ultra-High Tg Thermoset Fibers Obtained by Electrospinning of Functional Polynorbornenes" Nanomaterials 12, no. 6: 967. https://doi.org/10.3390/nano12060967
APA StyleCommarieu, B., Compaoré, M., de Boëver, R., Imbeault, R., Leprince, M., Martin, B., Perard, B., Qiu, W., & Claverie, J. P. (2022). Ultra-High Tg Thermoset Fibers Obtained by Electrospinning of Functional Polynorbornenes. Nanomaterials, 12(6), 967. https://doi.org/10.3390/nano12060967