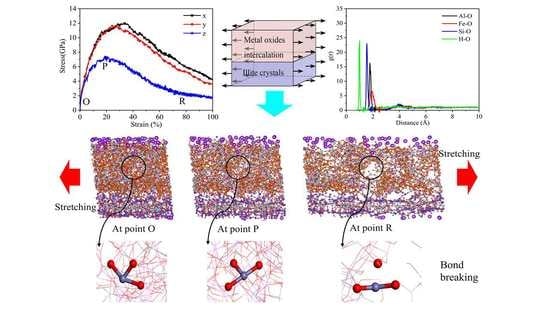
3.2.1. The Tensile Stress–Strain Curves
The hybrid illite-based structures were tensile along the
X-,
Y-, and
Z-directions (
Figure 4), and the corresponding tensile stress–strain curves are shown in
Figure 5. The typical stress–strain curves experienced several stages of linear increase, non-linear increase, and then decreased to the residual value with increasing strain. These stages, respectively, correspond to the elastic deformation, plastic hardening, and failure of the hybrid system. Interestingly, the stress–strain curves in the
X- and
Y- directions displayed the same tendency but showed a significant difference in the z-direction. The mechanical properties of the hybrid illite-based structures in the
X- and
Z-directions will be discussed in detail because of the similarity in the performances in the
X- and
Y-directions.
To further analyze the influence of different amorphous metal oxides on the mechanical properties of the hybrid illite-based structures, the tensile strength and Young’s moduli were calculated according to the tensile stress–strain curves. The Young’s moduli,
Yα, can be estimated by the elastic stage of illite-based structures:
where
and
are, respectively, the stress and strain in the
-direction.
The calculated tensile strength and the Young’s moduli are summarized in
Figure 6. The minimum tensile strength was obtained in the
Z-direction for each hybrid structure, showing very strong anisotropy. The tensile strength of structure D was only 4.67 GPa in the
Z-direction, which fluctuated around 7 GPa for the other structures. There were few differences in the tensile strength along the
X- and
Y-directions. In addition, the tensile strength of structure C was approximately 4 Gpa higher than that of the other structures in the
X- and
Y-directions. The variation in the Young’s moduli was similar to the tensile strength along the
X- and
Y-directions. However, the amorphous metal oxides had little impact on the Young’s moduli in the
Z-direction, implying the comparable energy barriers for the deformation of the hybrid structures.
3.2.2. Deformation and Failure Processes of the Hybrid Structures during Tensile
For the
X- and
Y-directions, the reason for the similarity of the stress–strain curves can attribute to the microstructure of the hybrid structures. Actually, the hybrid illite-based structures were composed of amorphous metal oxides and illite layers (as shown in
Figure 7a). The amorphous metal oxides intercalation exhibited isotropic mechanical properties due to the random arrangement of metal oxides (
Figure 6b). While illite belongs to T–O–T layered clay minerals, consisting of aluminum chains sandwiched between two silicon chains, which shows the similarity of the microstructure along the
X- and
Y-directions (
Figure 6c). Therefore, the overall hybrid illite-based structures exhibited similar mechanical properties in the
X- and
Y-directions.
As shown in
Figure 5a, the stress–strain variation tendencies of the hybrid structures were roughly the same. The stress required for the structural failure was the largest when the hybrid illite-based structure did not contain FeO(OH) (structure C). In addition, the peak and residual stresses of structure C were much higher than the other structures. The stretching processes of the structures along the
X-directions are shown in
Figure 8. Obviously, the main difference in the microstructure between structure C and the other structures lies in the type and content of chemical bonds in the amorphous metal oxide intercalation. The chemical bonding types of structures A, B, and D amorphous metal oxide intercalation mainly included Fe–O, O–H, OH…O, or Al–O bonds, which were Fe–O and Al–O bonds for structure C. Moreover, the amount of Fe–O bonds in the metal oxide intercalation of structure C was minimal. As mentioned previously, chemical bonds were the fundamental cause of the change in the tensile stress–strain curves. The main reason for the difference in the tensile stress–strain curves was the changes in the bonds. The normalized changes in the bonds during stretch along the
x-direction are listed in
Table 4, where the negative value means new bonds were formed.
Figure 8 exhibits that due to the crack propagation and failure of the hybrid illite-based structures, the number of broken bonds increased gradually during the stretching process (as shown in
Table 5). The sequences of broken bonds resulted from both the bond strength and the number of bonds for each type in the
x-direction. The largest percentage of hydrogen bonds (OH…O) was broken at the peak and residual states in the
x-direction. The percentage of broken Fe–O bonds was larger than that of the Al–O bonds, and the smallest percentage of Si–O bonds was broken during tension. It can be concluded that the influence of H–O bonds on the mechanical properties of the hybrid structures was negligible due to the lower percentage of bonds in the system and the bare variation of them. By comparing the bond-breaking ratios and the tensile stress–strain curves of structures A and B, it was obvious that the strength of the Al–O bonds was higher than that of the Fe–O bonds. Namely, the Al–O bonds were beneficial to the mechanical properties of the hybrid system. It should be pointed out that the Si–O bonds belonged to the illite crystal plate. The largest percentage of Si–O bonds was broken in structure C. This phenomenon resulted in the destruction of the illite crystal plate (
Figure 8c). However, the amorphous metal oxide intercalation in structure C was stabilized at the residual state. Consequently, the peak and the residual stresses of structure C were much higher than other structures because of the abundant Al–O bonds in the amorphous metal oxide intercalation. On the contrary, the microstructure of models A, B, and D were destroyed first within the amorphous metal oxide intercalation (
Figure 8). The reason was that the strength of the amorphous metal oxides intercalation was lower than that of the illite crystal plates when there include abundant Fe–O bonds; thus, the strength of the illite crystal plates was not destructed ahead of point P. This means the illite crystal plates determined the strength of these hybrid structures in the horizontal directions (
x- and
y-directions) if the amorphous metal oxide layer included many Fe–O bonds (namely, structures A, B, and D). Therefore, the stress–strain curves of A, B, and D share the same tendency.
The mechanical properties of the hybrid structures in the
z-direction were explored. The variations in the microstructures and the percentage of broken bonds during stretching the four hybrid structures along the
z-direction are displayed in
Figure 9 and
Table 5. Interfaces were formed when the amorphous metal oxide intercalation was intercalated in the interlayer of the illite crystal. An interesting phenomenon was observed, where a large number of chemical bonds were generated between the amorphous metal oxide intercalation and the original illite plate at interface 1 (
Figure 9). Many Al–O and Fe–O bonds were observed at interface 1 of structures A, B, and C, while much fewer Fe–O and OH…O bonds were detected for structure D. Few of the newly generated chemical bonds were discovered at interface 2, because of the separation layer of the potassium atoms.
In the process of stretching along the
z-direction, the OH…O bonds were destroyed first due to the fact of their low strength property.
Table 3 and
Table 6 illustrate that the differences in the total number of chemical bonds and the percentage of broken bonds for structures A and B are ignorable, resulting in the same performances during stretching. The broken Al–O and Fe–O bonds at interface 1 and within the amorphous metal oxide intercalation of structures A and B lead to deformation and failure (
Figure 9a,b). Additionally, interface 2 appears at a larger gap compared with interface 1, because the presence of the potassium atoms obstructs the formation of chemical bonds. Notably, new Si–O bonds were generated during stretching by employing the ReaxFF (
Table 6). The reason for this phenomenon was that the previously destroyed Si–O bonds of the illite plate caused the combination of silicon atoms with oxygen atoms in the amorphous metal oxide intercalation (
Figure 9). More Al–O and Fe–O bonds were generated in structure C at point P. Consequently, the deformation of structure C was mainly located in interface 2, and the deformations of the amorphous metal oxide intercalation r and interface 1 were negligible due to the high strength of the Al–O bonds. Different from that of structures A, B, and C, few Fe–O and OH…O bonds were detected at interface 1 of structure D. Therefore, interfaces 1 and 2 were the weak zones for the hybrid structure D, which disconnected the amorphous metal oxide intercalation from the illite plates during stretching (
Figure 9d). As a consequence, the minimum strength was observed in structure D (
Figure 9a).
The percentage of broken bonds along the Z-direction was much less than that of the X- and Y-directions, and the interfaces between the illite plate and the amorphous metal oxide intercalation were the weak zones when the hybrid structures were stretched in the z-direction, showing very strong anisotropy. Low load-bearing capacity because of the small bond content contributes to the weakest mechanical properties along the z-direction compared with that of the x- and y-directions.