Conversion of Carbon Dioxide into Methanol Using Cu–Zn Nanostructured Materials as Catalysts
Abstract
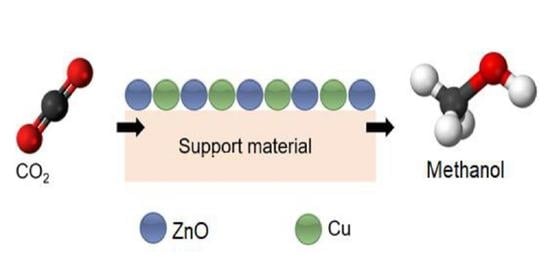
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Nanocomposites
2.2.1. ZnO Nanoparticle Synthesis
2.2.2. Cu Nanoparticle Synthesis
2.2.3. CuO/ZnO Bimetallic Nanoparticle Synthesis
2.2.4. Cu/ZnO Bimetallic Nanoparticle Synthesis
2.2.5. Cu/ZnO/Zeolite Nanocomposite Synthesis
2.2.6. Cu/ZnO/Polypyrrole Nanotube Synthesis
2.2.7. Cu/ZnO/Activated Carbon Synthesis
2.3. Characterisation of Catalysts
2.4. Catalytic Activity Test
3. Results and Discussion
3.1. Structural and Morphological Characterisation of Nanomaterials
3.2. Catalytic Activity of Nanomaterials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Alotaibi, M.A.; Din, I.U.; Alharthi, A.I.; Bakht, M.A.; Centi, G.; Shaharun, M.S.; Naeem, A. Green methanol synthesis by catalytic CO2 hydrogenation, decipehring the role of metal-metal interaction. Sustain. Chem. Pharm. 2021, 21, 100420. [Google Scholar] [CrossRef]
- Saeidi, S.; Najari, S.; Hessel, V.; Wilson, K.; Keil, F.J.; Concepción, P.; Suib, S.L.; Rodrigues, A.E. Recent advances in CO2 hydrogenation to value-added products—Current challenges and future directions. Prog. Energy Combust. Sci. 2021, 85, 100905. [Google Scholar] [CrossRef]
- Li, M.M.J.; Tsang, S.C.E. Bimetallic catalysts for green methanol production via CO2 and renewable hydrogen: A mini-review and prospects. Catal. Sci. Technol. 2018, 8, 3450–3464. [Google Scholar] [CrossRef]
- Ott, J.; Gronemann, V.; Pontzen, F.; Fiedler, E.; Grossmann, G.; Kersebohm, D.B.; Weiss, G.; Witte, C. Methanol-An Industrial Review. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH GmbH: Weinheim, Germany, 2012. [Google Scholar]
- Ertl, G.; Knözinger, H.; Weitkamp, J. Handbook of Heterogeneous Catalysis; Wiley-VCH GmbH: Weinheim, Germany, 2008; Volumes 1–5. [Google Scholar]
- Álvarez, A.; Bansode, A.; Urakawa, A.; Bavykima, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 hydrogenation Processes. Chem. Rev. 2017, 117, 9804–9838. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.E.; Delgado, J.J.; Mira, C.; Calvino, J.J.; Bernal, S.; Chiavassa, D.L.; Baltanás, M.A.; Bonivardi, A.L. The role of Pd-Ga bimetallic particles in the bifunctional mechanism of selective methanol synthesis via CO2 hydrogenation on a Pd/Ga2O3 catalyst. J. Catal. 2012, 292, 90–98. [Google Scholar] [CrossRef]
- Studt, F.; Sharafutdinov, I.; Abild-Pedersen, F.; Elkjaer, C.F.; Hummelshøj, J.S.; Dahl, S.; Chorkendorff, I.; Nørskov, J.K. Discovery of a Ni-Ga catalysts for carbon dioxide reduction to methanol. Nat. Chem. 2014, 6, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ain, N.; Ao, Q. Role of capping agents in the application of nanoparticles in biomedicine and environmental remediation: Recent trends and future prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent Materials for Carbon Dioxide Capture from Large Anthropogenic Point Sources. ChemSusChem 2009, 9, 796–854. [Google Scholar] [CrossRef]
- Alonso, A.; Moral-Vico, J.; Abo Markeb, A.; Busquets-Fité, M.; Komilis, D.; Puntes, V.; Sánchez, T.; Font, X. Critical review of existing nanomaterial adsorbents to capture carbon dioxide and methane. Sci. Total Environ. 2017, 595, 51–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stejskal, J.; Trchová, M. Conducting polypyrrole nanotubes: A review. Chem. Pap. 2018, 72, 1563–1595. [Google Scholar] [CrossRef]
- Peng, Y.; Qiu, L.; Pan, C.; Wang, C.; Shang, S.; Yan, F. Facile preparation of water dispersible polypyrrole nanotube-supported silver nanoparticles for hydrogen peroxide reduction and surface-enhanced Raman scattering. Electrochim. Acta 2012, 75, 399–405. [Google Scholar] [CrossRef]
- Yahya, N.; Nasir, A.M.; Daub, N.A.; Aziz, F.; Aizat, A.; Jaafar, J.; Lau, W.J.; Yusof, N.; Wan Salleh, W.N.; Fauzi Ismaul, A.; et al. Visible light-driven perovskite-based photocatalyst for wastewater treatment. In Handbook of Smart Photocatalytic Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 265–302. [Google Scholar]
- Houshiar, M.; Zebhi, F.; Razi, Z.J.; Alidoust, A.; Askari, Z. Synthesis of cobalt ferrite (CoFe2O4) nanoparticles using combustion, coprecipitation, and precipitation methods: A comparison study of size, structural and magnetic properties. J. Magn. Magn. Mater. 2014, 371, 43–48. [Google Scholar] [CrossRef]
- Kawano, T.; Imai, H. Fabrication of ZnO Nanoparticles with Various Aspect Ratios through Acidic and Basic Routes. Cryst. Growth Des. 2006, 6, 1054–1056. [Google Scholar] [CrossRef]
- Dung Dang, T.M.; Thu Le, T.T.; Fribourg-Blanc, E.; Chien Dang, M. Synthesis and optical properties of copper nanoparticles prepared by a chemical reduction method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2011, 2, 015009. [Google Scholar] [CrossRef]
- Witoon, T.; Permsirivanich, T.; Donphai, W.; Jaree, A.; Chareonpanich, M. CO2 hydrogenation to methanol over Cu/ZnO nanocatalysts prepared via a chitosan-assisted co-precipitation method. Fuel Process. Technol. 2013, 116, 72–78. [Google Scholar] [CrossRef]
- Medina-Ramírez, I.E.; Arzate-Cardenas, M.A.; Mojarro-Olmos, A.; Romo-López, M.A. Synthesis, characterization, toxicological and antibacterial activity evaluation of Cu@ZnO nanocomposites. Ceram. Int. 2019, 45, 17476–17488. [Google Scholar] [CrossRef]
- Taha, A.; Da’na, E.; Hassanin, H.A. Modified activated carbon loaded with bio-synthesized Ag/ZnO nanocomposite and its application for the removel of Cr (VI) ions from aqueous solution. Surf. Interfaces 2021, 23, 100928. [Google Scholar] [CrossRef]
- Anbu, P.; Jayanthi, S.; Velusamy, P. Characterization of nanoparticles using nano-analytical techniques. In Nanoparticles in Analytical and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Mercury-Crystal Structure, Visualisation, Exploration and Analysis Made Easy. Available online: https://www.ccdc.cam.ac.uk/Community/csd-community/freemercury/ (accessed on 21 February 2022).
- Salavati-Niasari, M.; Davar, F.; Mir, N. Synthesis and characterization of metallic copper nanoparticles via thermal decomposition. Int. J. Appl. Adv. Sci. Res. 2016, 1, 128–133. [Google Scholar] [CrossRef]
- Shoja Razavi, R.; Lonhman-Estarki, M.R. Synthesis and Characterizations of Copper Oxide Nanoparticles within Zeolite Y. J. Clust. Sci. 2012, 23, 1097–1106. [Google Scholar] [CrossRef]
- Kopecká, J.; Kopecký, D.; Vrňata, M.; Fitl, P.; Stejskal, J.; Trchová, M.; Bober, P.; Morávková, Z.; Prokeš, J.; Sapurina, I. Polypyrrole nanotubes: Mechanism of formation. RSC Adv. 2014, 4, 1551–1558. [Google Scholar] [CrossRef]
- Cho, K.; Ryoo, R.; Asahina, S.; Xiao, C.; Klingstedt, M.; Umemura, A.; Anderson, M.W.; Terasaki, O. Mesopore generation by organosilane surfactant during LTA zeolite crystallization, investigated by high-resolution SEM and Monte Carlo simulation. SolidState Sci. 2011, 13, 750–756. [Google Scholar] [CrossRef]
- Stangeland, K.; Li, H.; Yu, Z. CO2 hydrogenation to methanol: The structure-activity relationships of different catalyst systems. Energ. Ecol. Environ. 2020, 5, 272–285. [Google Scholar] [CrossRef] [Green Version]
- Behrens, M.; Studt, F.; Kasatkin, I.; Kühl, S.; Hävecker, M.; Abild-Pedersen, F.; Zander, S.; Girgsdies, F.; Kurr, P.; Kniep, B.; et al. The Active Site of Methanol Synthesis over Cu/ZnO/Al2O3 Industrial Catalysts. Science 2012, 759, 893–898. [Google Scholar]
- Dasireddy, V.D.B.C.; Likozar, B. The role of copper oxidation state in Cu/ZnO/Al2O3 catalysts in CO2 hydrogenation and methanol productivity. Renew. Energy 2019, 140, 452–460. [Google Scholar] [CrossRef]
- Dong, X.; Li, F.; Zhao, N.; Xiao, F.; Wang, J.; Tan, Y. CO2 hydrogenation to methanol over Cu/ZnO/ZrO2 catalysts prepared by precipitation-reduction method. Appl. Catal. B Environ. 2016, 191, 8–17. [Google Scholar] [CrossRef]
- Schumann, J.; Tarasov, A.; Thomas, N.; Schlögl, R.; Behrens, M. Cu, Zn-based catalysts for methanol synthesis: On the effect of calcination conditions and the part of residual carbonates. Appl. Catal. A Gen. 2016, 516, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Lei, H.; Nie, R.; Wu, G.; Hou, Z. Hydrogenation of CO2 to CH3OH over Cu/ZnO catalysts with different ZnO morphology. Fuel 2015, 154, 161–166. [Google Scholar] [CrossRef]
- Studt, F.; Behrens, M.; Kunkes, E.L.; Thomas, N.; Zander, S.; Tarasov, A.; Schumann, J.; Frei, E.; Varley, J.B.; Abild-Pedersen, F.; et al. The Mechanism of CO and CO2 Hydrogenation to Methanol over Cu-Based Catalysts. ChemCatChem 2015, 7, 1105–1111. [Google Scholar] [CrossRef] [Green Version]
- Martin, O.; Mondelli, C.; Curulla-Ferré, D.; Drouilly, C.; Hauert, R.; Pérez-Ramírez, J. Zinc-Rich Copper Catalysts Promoted by Gold for Methanol Synthesis. ACS Catal. 2015, 5, 5607–5616. [Google Scholar] [CrossRef]
- Huang, C.; Wen, J.; Sun, Y.; Zhang, M.; Bao, Y.; Zhang, Y.; Liang, L.; Fu, M.; Wu, J.; Ye, D.; et al. CO2 hydrogenation to methanol over Cu/ZnO plate model catalysts: Effects of reducing gas induced Cu nanoparticle morphology. Chem. Engin. J. 2019, 374, 221–230. [Google Scholar] [CrossRef]
- Sun, Y.; Huang, C.; Chen, L.; Zhang, Y.; Fu, M.; Wu, J.; Ye, D. Active site structure study of Cu/Plate ZnO model catalysts for CO2 hydrogenation to methanol under the real reaction conditions. J. CO2 Util. 2020, 37, 55–64. [Google Scholar] [CrossRef]
- Niu, J.; Liu, H.; Fan, B.; Qi, W.; Ran, J. Comprehensive review of Cu-based CO2 hydrogenation to CH3OH: Insights from experimental work and theoretical analysis. Int. J. Hydrogen Energy 2022, 47, 9183–9200. [Google Scholar] [CrossRef]
- Xiuyi, X.; Zhang, H. Kinetically relevant variation triggered by hydrogen pressure: A mechanistic case study of CO2 hydrogenation to methanol over Cu/ZnO. J. Catal. 2022, 406, 145–156. [Google Scholar]










| Element | Weight (%) | Atomic (%) |
|---|---|---|
| O | 46.93 | 70.51 |
| Al | 9.67 | 8.61 |
| Si | 9.60 | 8.21 |
| Cu | 21.84 | 8.26 |
| Zn | 11.98 | 4.40 |
| Element | Weight (%) | Atomic (%) |
|---|---|---|
| O | 20.90 | 21.05 |
| C | 53.94 | 72.39 |
| Na | 0.51 | 0.36 |
| Cu | 16.97 | 4.31 |
| Zn | 7.68 | 1.89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, A.C.; Moral-Vico, J.; Abo Markeb, A.; Sánchez, A. Conversion of Carbon Dioxide into Methanol Using Cu–Zn Nanostructured Materials as Catalysts. Nanomaterials 2022, 12, 999. https://doi.org/10.3390/nano12060999
García AC, Moral-Vico J, Abo Markeb A, Sánchez A. Conversion of Carbon Dioxide into Methanol Using Cu–Zn Nanostructured Materials as Catalysts. Nanomaterials. 2022; 12(6):999. https://doi.org/10.3390/nano12060999
Chicago/Turabian StyleGarcía, Anna Carrasco, Javier Moral-Vico, Ahmad Abo Markeb, and Antoni Sánchez. 2022. "Conversion of Carbon Dioxide into Methanol Using Cu–Zn Nanostructured Materials as Catalysts" Nanomaterials 12, no. 6: 999. https://doi.org/10.3390/nano12060999
APA StyleGarcía, A. C., Moral-Vico, J., Abo Markeb, A., & Sánchez, A. (2022). Conversion of Carbon Dioxide into Methanol Using Cu–Zn Nanostructured Materials as Catalysts. Nanomaterials, 12(6), 999. https://doi.org/10.3390/nano12060999