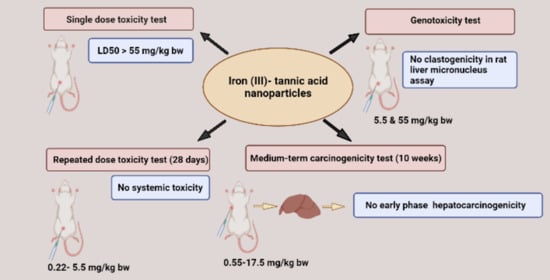
Assessment of Systemic Toxicity, Genotoxicity, and Early Phase Hepatocarcinogenicity of Iron (III)-Tannic Acid Nanoparticles in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Iron (III)—Tannic Acid Nanoparticles
2.3. Animal Studies
2.4. Single Dose Toxicity Test
2.5. Repeated Dose Toxicity Test
2.6. Clastogenicity Test
2.7. Medium-Term Liver Carcinogenicity Test
2.8. Measurement of Serum and Liver Iron Status Parameters
2.9. Determination of Antioxidant Parameters in Rat Livers
2.10. Statistical Analysis
3. Results
3.1. Single and Repeated Dose Toxicity Levels of Iron (III)–Tannic Acid Nanoparticles in Rats
3.2. Clastogenic Effect of Iron-Tannic Molecular Nanoparticles in Rats
3.3. Carcinogenicity of Iron (III)–Tannic Acid Nanoparticles in Rats
3.4. Effect of Iron (III)–Tannic Acid Nanoparticles on Some Biochemical Parameters in Serum and Livers of Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Daher, S.; Massarwa, M.; Benson, A.A.; Khoury, T. Current and future treatment of hepatocellular carcinoma: An updated comprehensive review. J. Clin. Transl. Hepatol. 2018, 6, 69. [Google Scholar] [CrossRef] [Green Version]
- Ghanaati, H.; Alavian, S.M.; Jafarian, A.; Daryani, N.E.; Nassiri-Toosi, M.; Jalali, A.H.; Shakiba, M. Imaging and imaging-guided interventions in the diagnosis and management of hepatocellular carcinoma (HCC)-review of evidence. Iran J. Radiol. 2012, 9, 167. [Google Scholar] [CrossRef] [Green Version]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, C.O.; Pinho, J.O.; Lopes, J.M.; Almeida, A.J.; Gaspar, M.M.; Reis, C. Current trends in cancer nanotheranostics: Metallic, polymeric, and lipid-based systems. Pharmaceutics 2019, 11, 22. [Google Scholar] [CrossRef] [Green Version]
- Alshehri, S.; Imam, S.S.; Rizwanullah, M.; Akhter, S.; Mahdi, W.; Kazi, M.; Ahmad, J. Progress of cancer nanotechnology as diagnostics, therapeutics, and theranostics nanomedicine: Preclinical promise and translational challenges. Pharmaceutics 2021, 13, 24. [Google Scholar] [CrossRef]
- Ruman, U.; Fakurazi, S.; Masarudin, M.J.; Hussein, M.Z. Nanocarrier-based therapeutics and theranostics drug delivery systems for next generation of liver cancer nanodrug modalities. Int. J. Nanomed. 2020, 15, 1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, P.; Shi, X.; Zhong, S.; Peng, Y.; Qi, Y.; Ding, J.; Zhou, W. Metal-phenolic networks for cancer theranostics. Biomater. Sci. 2021, 9, 2825–2849. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Geng, H.; Yu, Q.; Hao, J.; Cui, J. Polyphenol-based particles for theranostics. Theranostics 2019, 9, 3170–3190. [Google Scholar] [CrossRef] [PubMed]
- Saowalak, K.; Titipun, T.; Somchai, T.; Chalermchai, P. Iron (III)-tannic molecular nanoparticles enhance autophagy effect and T 1 MRI contrast in liver cell lines. Sci. Rep. 2018, 8, 6647. [Google Scholar] [CrossRef]
- Phatruengdet, T.; Intakhad, J.; Tapunya, M.; Chariyakornkul, A.; Hlaing, C.B.; Wongpoomchai, R.; Pilapong, C. MRI contrast enhancement of liver pre-neoplasia using iron–tannic nanoparticles. RSC Adv. 2020, 10, 35419–35425. [Google Scholar] [CrossRef]
- Jodynis-Liebert, J.; Kujawska, M. Biphasic dose-response induced by phytochemicals: Experimental evidence. J. Clin. Med. 2020, 9, 718. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, S.; Kinoshita, A.; Puatanachokchai, R.; Kushida, M.; Wanibuchi, H.; Morimura, K. Hormesis and dose–response-mediated mechanisms in carcinogenesis: Evidence for a threshold in carcinogenicity of non-genotoxic carcinogens. Carcinogenesis 2005, 26, 1835–1845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaiserman, A.; Cuttler, J.M.; Socol, Y. Low-dose ionizing radiation as a hormetin: Experimental observations and therapeutic perspective for age-related disorders. Biogerontology 2021, 22, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Phatruengdet, T.; Khuemjun, P.; Intakhad, J.; Krunchanuchat, S.; Chariyakornkul, A.; Wongpoomchai, R.; Pilapong, C. Pharmacokinetic/pharmacodynamic determinations of iron-tannic molecular nanoparticles with its implication in MR imaging and enhancement of liver clearance. Nanotheranostics 2022, 6, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Chariyakornkul, A.; Punvittayagul, C.; Taya, S.; Wongpoomchai, R. Inhibitory effect of purple rice husk extract on AFB 1-induced micronucleus formation in rat liver through modulation of xenobiotic metabolizing enzymes. BMC Compl. Altern. Med. 2019, 19, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dokkaew, A.; Punvittayagul, C.; Insuan, O.; Limtrakul Dejkriengkraikul, P.; Wongpoomchai, R. Protective effects of defatted sticky rice bran extracts on the early stages of hepatocarcinogenesis in rats. Molecules 2019, 24, 2142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koonyosying, P.; Kongkarnka, S.; Uthaipibull, C.; Svasti, S.; Fucharoen, S.; Srichairatanakool, S. Green tea extract modulates oxidative tissue injury in beta-thalassemic mice by chelation of redox iron and inhibition of lipid peroxidation. Biomed. Pharmacother. 2018, 108, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, I.; Mannervik, B. Glutathione reductase. Methods Enzymol. 1985, 113, 484–490. [Google Scholar] [CrossRef]
- Nagalakshmi, N.; Prasad, M.N. Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci. 2001, 160, 291–299. [Google Scholar] [CrossRef]
- Punvittayagul, C.; Wongpoomchai, R.; Taya, S.; Pompimon, W. Effect of pinocembrin isolated from Boesenbergia pandurata on xenobiotic-metabolizing enzymes in rat liver. Drug Metab. Lett. 2011, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Krishna, G.; Hayashi, M. In vivo rodent micronucleus assay: Protocol, conduct and data interpretation. Mutat. Res. 2000, 455, 155–166. [Google Scholar] [CrossRef]
- Ito, N.; Tamano, S.; Shirai, T. A medium-term rat liver bioassay for rapid in vivo detection of carcinogenic potential of chemicals. Cancer Sci. 2003, 94, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, L.; Mettenbrink, E.M.; DeAngelis, P.L.; Wilhelm, S. Nanoparticle toxicology. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 269–289. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.; Larsen, S.; Spliid, H.; Christensen, N.D. Multivariate statistical analysis of organ weights in toxicity studies. Toxicology 1999, 136, 67–77. [Google Scholar] [CrossRef]
- Wang, R.; Song, B.; Wu, J.; Zhang, Y.; Chen, A.; Shao, L. Potential adverse effects of nanoparticles on the reproductive system. Int. J. Nanomed. 2018, 13, 8487–8506. [Google Scholar] [CrossRef] [Green Version]
- Rudmann, D.G.; Foley, G.L. Female Reproductive System. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology; Haschek, W.M., Rousseaux, C.G., Wallig, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 3, pp. 2599–2633. [Google Scholar]
- Creasy, D.M.; Chapin, R.E. Male reproductive system. In Haschek and Rousseaux’s Handbook of Toxicologic Pathology, 3rd ed.; Haschek, W.M., Rousseaux, C.G., Wallig, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 3, pp. 2493–2598. [Google Scholar]
- Sharma, V.; Aneja, B.; Yata, V.K.; Malakar, D.; Mohanty, A.K. Systemic nanotoxicity and its assessment in animal models. In Nanopharmaceuticals: Principles and Applications; Yata, V.R.S., Dasgupta, N., Lichtfouse, E., Eds.; Springer: Cham, Switzerland; New York, NY, USA, 2020; Volume 3, pp. 201–243. [Google Scholar]
- Katsnelson, B.A.; Degtyareva, T.D.; Minigalieva, I.I.; Privalova, L.I.; Kuzmin, S.V.; Yeremenko, O.S.; Kireyeva, E.P.; Sutunkova, M.P.; Valamina, I.I.; Khodos, M.Y.; et al. Subchronic systemic toxicity and bioaccumulation of Fe3O4 nano- and microparticles following repeated intraperitoneal administration to rats. Int. J. Toxicol. 2011, 30, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.J.; Frazer, D.M. Current understanding of iron homeostasis. Am. J. Clin. Nutr. 2017, 106, 1559S–1566S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrishtop, V.V.; Mironov, V.A.; Prilepskii, A.Y.; Nikonorova, V.G.; Vinogradov, V.V. Organ-specific toxicity of magnetic iron oxide-based nanoparticles. Nanotoxicology 2020, 15, 167–204. [Google Scholar] [CrossRef] [PubMed]
- Phiwchai, I.; Yuensook, W.; Sawaengsiriphon, N.; Krungchanuchat, S.; Pilapong, C. Tannic acid (TA): A molecular tool for chelating and imaging labile iron. Eur. J. Pharm. Sci. 2018, 114, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Saul, N.; Pietsch, K.; Menzel, R.; Sturzenbaum, S.R.; Steinberg, C.E. The longevity effect of tannic acid in Caenorhabditis elegans: Disposable Soma meets hormesis. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 626–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iavicoli, I.; Leso, V.; Fontana, L.; Calabrese, E.J. Nanoparticle exposure and hormetic dose-responses: An update. Int. J. Mol. Sci. 2018, 19, 805. [Google Scholar] [CrossRef] [Green Version]
- Perry, S. Proteinuria in the Wistar rat. J. Pathol. Bacteriol. 1965, 89, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Alt, J.M.; Hackbarth, H.; Deerberg, F.; Stolte, H. Proteinuria in rats in relation to age-dependent renal changes. Lab. Anim. 1980, 14, 95–101. [Google Scholar] [CrossRef] [Green Version]
- Yin, S.; Liu, J.; Kang, Y.; Lin, Y.; Li, D.; Shao, L. Interactions of nanomaterials with ion channels and related mechanisms. Br. J. Pharmacol. 2019, 176, 3754–3774. [Google Scholar] [CrossRef]
- Tanaka, T.; Shimizu, M.; Kochi, T.; Moriwaki, H. Chemical-induced carcinogenesis. J. Exp. Clin. Med. 2013, 5, 203–209. [Google Scholar] [CrossRef]
- Khan, N.S.; Ahmad, A.; Hadi, S.M. Anti-oxidant, pro-oxidant properties of tannic acid and its binding to DNA. Chem.-Biol. Interact 2000, 125, 177–189. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, J.; Chen, N.G.; Shi, Z.; Qiu, J.; He, C.; Chen, M. Recent advances in anticancer activities and drug delivery systems of tannins. Med. Res. Rev. 2017, 37, 665–701. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baud, O.; Greene, A.E.; Li, J.; Wang, H.; Volpe, J.J.; Rosenberg, P.A. Glutathione peroxidase-catalase cooperativity is required for resistance to hydrogen peroxide by mature rat oligodendrocytes. J. Neurosci. 2004, 24, 1531–1540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canli, E.G.; Ila, H.B.; Canli, M. Response of the antioxidant enzymes of rats following oral administration of metal-oxide nanoparticles (Al2O3, CuO, TiO2). Environ. Sci. Pollut. Res. Int. 2019, 26, 938–945. [Google Scholar] [CrossRef] [PubMed]





| Parameters | Fe-TA NPs Treatment (mg/kg bw) | |||||
|---|---|---|---|---|---|---|
| 0 | 0.22 | 1.1 | 5.5 | Satellite | ||
| 0 | 5.5 | |||||
| Female | ||||||
| Food intake (g/day) | 14.3 ± 2.0 | 15.9 ± 2.3 | 14.5 ± 1.3 | 15.1 ± 1.8 | 15.4 ± 1.8 | 16.4 ± 2.1 |
| Water intake (mL/day) | 18.9 ± 3.2 | 19.7 ± 5.5 | 20.8 ± 5.1 | 21.0 ± 3.7 | 22.5 ± 1.9 | 19.0 ± 2.4 |
| IBW (g) | 120 ± 6.12 | 120 ± 8.66 | 121 ± 5.48 | 121 ± 4.18 | 120 ± 7.07 | 121 ± 2.24 |
| FBW (g) | 187 ± 4.47 | 193 ± 11.0 | 188 ± 17.2 | 193 ± 9.08 | 214 ± 15.5 | 204 ± 9.46 |
| Liver (g) | 6.36 ± 0.54 | 6.88 ± 0.30 | 6.53 ± 0.43 | 6.81 ± 0.47 | 6.94 ± 0.81 | 6.99 ± 0.48 |
| Spleen (g) | 0.37 ± 0.04 | 0.40 ± 0.03 | 0.36 ± 0.06 | 0.45 ± 0.04 | 0.39 ± 0.07 | 0.38 ± 0.03 |
| Kidneys (g) | 1.50 ± 0.06 | 1.57 ± 0.12 | 1.52 ± 0.14 | 1.49 ± 0.09 | 1.57 ± 0.17 | 1.51 ± 0.10 |
| Lungs (g) | 0.97 ± 0.12 | 0.89 ± 0.10 | 0.88 ± 0.08 | 0.90 ± 0.02 | 0.92 ± 0.14 | 0.83 ± 0.05 |
| Heart (g) | 0.60 ± 0.02 | 0.59 ± 0.05 | 0.56 ± 0.07 | 0.58 ± 0.07 | 0.62 ± 0.05 | 0.61 ± 0.03 |
| Thymus (g) | 0.38 ± 0.08 | 0.46 ± 0.03 | 0.43 ± 0.08 | 0.43 ± 0.10 | 0.46 ± 0.04 | 0.31 ± 0.04 |
| Pancreas (g) | 0.62 ± 0.16 | 0.68 ± 0.25 | 0.72 ± 0.29 | 0.92 ± 0.21 | 0.57 ± 0.07 | 0.69 ± 0.08 |
| Brain (g) | 1.60 ± 0.08 | 1.66 ± 0.06 | 1.64 ± 0.04 | 1.65 ± 0.06 | 1.36 ± 0.13 | 1.18 ± 0.03 # |
| Adrenal (mg) | 64.0 ± 18.3 | 55.5 ± 9.06 | 60.8 ± 11.7 | 58.3 ± 3.61 | 64.9 ± 4.78 | 71.1 ± 13.8 |
| Ovary (mg) | 69.5 ± 15.4 | 91.1 ± 11.2 | 89.0 ± 12.5 | 110 ± 7.81 * | 103 ± 31.2 | 80.7 ± 15.3 |
| Male | ||||||
| Food intake (g/day) | 23.9 ± 2.6 | 26.9 ± 6.5 | 25.4 ± 5.2 | 23.4 ± 1.6 | 23.5 ± 1.7 | 23.8 ± 2.9 |
| Water intake (ml/day) | 27.9 ± 8.9 | 32.6 ± 6.8 | 32.4 ± 8.2 | 33.3 ± 5.1 | 30.0 ± 4.25 | 29.7 ± 4.22 |
| IBW (g) | 180 ± 12.3 | 180 ± 9.35 | 180 ± 6.12 | 180 ± 0.00 | 180 ± 14.1 | 180 ± 9.35 |
| FBW (g) | 321 ± 30.9 | 338 ± 23.9 | 314 ± 25.1 | 316 ± 9.62 | 365 ± 32.4 | 363 ± 18.6 |
| Liver (g) | 11.0 ± 1.33 | 12.0 ± 1.17 | 11.7 ± 1.56 | 11.0 ± 0.83 | 11.1 ± 1.91 | 11.0 ± 0.57 |
| Spleen (g) | 0.63 ± 0.14 | 0.64 ± 0.06 | 0.63 ± 0.07 | 0.67 ± 0.05 | 0.65 ± 0.14 | 0.66 ± 0.04 |
| Kidneys (g) | 2.46 ± 0.21 | 2.54 ± 0.15 | 2.62 ± 0.28 | 2.34 ± 0.08 | 2.38 ± 0.21 | 2.47 ± 0.31 |
| Lungs (g) | 1.18 ± 0.12 | 1.29 ± 0.18 | 1.38 ± 0.32 | 1.21 ± 0.15 | 1.13 ± 0.09 | 1.29 ± 0.20 |
| Heart (g) | 0.91 ± 0.15 | 0.93 ± 0.13 | 0.89 ± 0.07 | 0.88 ± 0.05 | 0.91 ± 0.05 | 0.96 ± 0.09 |
| Thymus (g) | 0.67 ± 0.14 | 0.67 ± 0.23 | 0.62 ± 0.09 | 0.62 ± 0.14 | 0.55 ± 0.14 | 0.48 ± 0.10 |
| Pancreas (g) | 0.79 ± 0.14 | 0.94 ± 0.22 | 0.87 ± 0.19 | 0.76 ± 0.22 | 0.73 ± 0.24 | 0.97 ± 0.17 |
| Brain (g) | 1.92 ± 0.09 | 1.85 ± 0.08 | 1.90 ± 0.09 | 1.82 ± 0.06 | 1.86 ± 0.10 | 1.84 ± 0.08 |
| Adrenal (mg) | 58.90 ± 6.32 | 55.00 ± 7.36 | 63.94 ± 17.90 | 64.36 ± 12.54 | 60.60 ± 10.06 | 66.58 ± 12.35 |
| Testes (g) | 3.34 ± 0.26 | 3.20 ± 0.23 | 3.30 ± 0.17 | 3.10 ± 0.19 | 3.65 ± 0.11 | 3.64 ± 0.17 |
| Prostate (g) | 0.39 ± 0.12 | 0.38 ± 0.09 | 0.41 ± 0.04 | 0.31 ± 0.06 | 0.44 ± 0.09 | 0.39 ± 0.05 |
| Epididymis (g) | 0.79 ± 0.12 | 0.78 ± 0.18 | 0.66 ± 0.12 | 0.63 ± 0.11 | 1.05 ± 0.07 | 1.03 ± 0.09 |
| Seminal (g) | 0.72 ± 0.11 | 0.76 ± 0.09 | 0.95 ± 0.09 * | 0.84 ± 0.08 | 0.89 ± 0.19 | 0.81 ± 0.25 |
| Parameters | Normal Range | Fe-TA NPs Treatment (mg/kg bw) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 0.22 | 1.1 | 5.5 | Satellite | |||
| 0 | 5.5 | ||||||
| Female | |||||||
| RBC (×106 cell/µL) | 4.6–9.2 | 7.3 ± 0.3 | 6.9 ± 0.6 | 7.6 ± 0.5 | 7.3 ± 0.4 | 8.5 ± 0.1 | 8.7 ± 0.6 |
| Hemoglobin (g/dL) | 11–19.2 | 15.7 ± 0.4 | 14.4 ± 1.2 | 16.1 ± 0.8 | 15.2 ± 0.8 | 18.1 ± 0.6 | 18.8 ± 1.1 |
| Hematocrit (%) | 36–53 | 48 ± 0.6 | 44 ± 3.2 | 49 ± 2.3 | 45.9 ± 2.0 | 52.9 ± 1.3 | 52.9 ± 2.7 |
| MCV (fL) | 48–70 | 66 ± 2.4 | 64 ± 1.3 | 65 ± 2.7 | 63 ± 1.1 | 62 ± 2.0 | 61 ± 2.0 |
| MCH (pg) | 16.0–23.1 | 21.7 ± 0.5 | 21.0 ± 0.8 | 21.3 ± 0.9 | 20.9 ± 0.8 | 21.3 ± 0.8 | 21.6 ± 0.6 |
| MCHC (g/dL) | 28.2–34.1 | 33.1 ± 0.7 | 32.7 ± 0.6 | 33.0 ± 0.3 | 33.2 ± 0.7 | 34.2 ± 0.7 | 35.5 ± 0.2 |
| RDW (%) | 10.0–16.0 | 12.2 ± 0.3 | 12.3 ± 0.8 | 12.2 ± 0.4 | 12.7 ± 0.5 | 12.6 ± 1.0 | 13.0 ± 0.9 |
| Reticulocytes (%) | 1.7–4.9 | 4.9 ± 1.6 | 3.8 ± 1.4 | 3.9 ± 0.8 | 3.7 ± 1.4 | 3.0 ± 1.0 | 2.9 ± 0.9 |
| WBC (×103 cell/mm3) | 2–17 | 4 ± 1 | 3 ± 1 | 5 ± 1 | 5 ± 0 | 5 ± 1 | 4 ± 1 |
| Neutrophil (%) | 10–30 | 12 ± 3.9 | 12 ± 3.4 | 15 ± 10.4 | 12 ± 10.3 | 10 ± 1.0 | 16 ± 2.8 |
| Lymphocyte (%) | 65–85 | 83 ± 4.3 | 83 ± 4.30 | 79 ± 7.9 | 84 ± 8.5 | 85 ± 2.1 | 77.5 ± 2.4 |
| Monocyte (%) | 0–5 | 4 ± 2.5 | 4.8 ± 2.5 | 6 ± 2.95 | 4 ± 2.1 | 5 ± 0.1 | 7 ± 1.5 |
| Eosinophil (%) | 0–6 | 1 ± 1.2 | 0.4 ± 0.89 | 0.2 ± 0.45 | 0.0 ± 0.0 | 0.3 ± 0.5 | 0.0 ± 0.0 |
| Basophil (%) | 0–1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| PLT (×103 cell/mm3) | 500–1300 | 787 ± 67 | 480 ± 337 | 762 ± 77 | 67.7 ± 206 | 793 ± 38 | 625 ± 315 |
| Male | |||||||
| RBC (×106 cell/µL) | 4.6–9.2 | 5.8 ± 0.2 | 5.8 ± 0.3 | 6.0 ± 0.2 | 5.8 ± 0.2 | 6.7 ± 0.8 | 6.4 ± 0.9 |
| Hemoglobin (g/dL) | 11–19.2 | 16.7 ± 0.3 | 16.7 ± 0.3 | 16.7 ± 0.4 | 16.1 ± 0.5 | 15.1 ± 0.6 | 14.7 ± 0.5 |
| Hematocrit (%) | 36–53 | 49.1 ± 1.4 | 48.3 ± 1.6 | 49.3 ± 1.4 | 46.8 ± 1.1 | 45.4 ± 1.8 | 44.0 ± 1.6 |
| MCV (fL) | 48–70 | 84.0 ± 1.6 | 83.2 ± 2.6 | 82.4 ± 1.9 | 81.1 ± 1.8 | 68.1 ± 6.3 | 70.2 ± 8.4 |
| MCH (pg) | 16.0–23.1 | 28.6 ± 0.8 | 28.7 ± 1.0 | 28.0 ± 0.5 | 28.0 ± 0.5 | 22.6 ± 2.1 | 23.5 ± 2.8 |
| MCHC (g/dL) | 28.2–34.1 | 34.0 ± 0.5 | 34.5 ± 0.4 | 33.9 ± 0.5 | 34.5 ± 0.3 | 33.4 ± 0.3 | 33.4 ± 0.1 |
| RDW (% | 10.0–16.0 | 10.9 ± 0.3 | 11.1 ± 0.7 | 11.2 ± 0.5 | 11.3 ± 0.6 | 14.6 ± 3.6 | 12.8 ± 3.4 |
| Reticulocytes (%) | 1.7–4.9 | 3.2 ± 2.1 | 3.0 ± 1.2 | 5.0 ± 1.2 | 5.4 ± 2.0 | 2.1 ± 1.7 | 2.3 ± 1.0 |
| WBC (×103 cell/mm3) | 2–17 | 6 ± 1 | 6 ± 2 | 7 ± 1 | 7 ± 2 | 5 ± 3 | 5 ± 2 |
| Neutrophil (%) | 10–30 | 7 ± 2.1 | 10 ± 0.5 | 14 ± 3.5 * | 15 ± 5.2 * | 17 ± 0.9 | 15 ± 6.0 |
| Lymphocyte (%) | 65–85 | 89 ± 2.2 | 87 ± 0.9 | 86 ± 3.5 | 85 ± 5.2 | 82 ± 1.1 | 83 ± 6.3 |
| Monocyte (%) | 0–5 | 5 ± 0.8 | 3 ± 1.1 * | 0 ± 0.0 * | 0 ± 0.0 * | 1 ± 0.5 | 2 ± 0.5 |
| Eosinophil (%) | 0–6 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Basophil (%) | 0–1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| PLT (×103 cell/mm3) | 500–1300 | 770 ± 177 | 802 ± 151 | 770 ± 103 | 915 ± 216 | 731 ± 65.2 | 590 ± 129 |
| Parameters | Normal Range | Fe-TA NPs Treatment (mg/kg bw) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 0.22 | 1.1 | 5.5 | Satellite | |||
| 0 | 5.5 | ||||||
| Female | |||||||
| Glucose (mg/dL) | 85–132 | 129 ± 14 | 144 ± 25 | 142 ± 13 | 167 ± 20 | 61 ± 21 | 173 ± 61 |
| Cholesterol (mg/dL) | 40–130 | 63 ± 10 | 70 ± 9 | 65 ± 8 | 67 ± 12 | 69 ± 4 | 68 ± 10 |
| Triglyceride (mg/dL) | 80–190 | 55 ± 12 | 49 ± 30 | 39± 8 | 41 ± 13 | 80 ± 11 | 55 ± 20 |
| BUN (mg/dL) | 10–21 | 24 ± 2 | 22 ± 3 | 18 ± 3 * | 19 ± 2 * | 25 ± 5 | 22 ± 2 |
| Creatinine (mg/dL) | 0.5–1.0 | 0.3 ± 0.1 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.3± 0.0 | 0.3 ± 0.1 |
| Total protein (g/dL) | 6.3–8.6 | 7.1 ± 0.2 | 6.8 ± 0.6 | 7.0 ± 0.2 | 7.1 ± 0.3 | 8.5 ± 0.3 | 9.0 ± 0.5 |
| Albumin (g/dL) | 3.3–4.9 | 4.6 ± 0.1 | 4.3 ± 0.3 | 4.4 ± 0.2 | 4.4 ± 0.1 | 4.7 ± 0.2 | 4.7 ± 0.2 |
| AST (U/L) | 39–92 | 37 ± 17 | 42 ± 19 | 34 ± 7 | 28 ± 5.9 | 37 ± 12 | 42 ± 14 |
| ALT (U/L) | 17–50 | 117 ± 42 | 102 ± 16 | 122 ± 31 | 95 ± 26 | 96 ± 16 | 103 ± 20 |
| ALP (U/L) | 39–216 | 49 ± 7 | 55 ± 10 | 51 ± 10 | 54 ± 17 | 46 ± 6 | 33 ± 2 |
| Total bil (mg/dL) | 0.05–0.17 | 0.14 ± 0.02 | 0.09 ± 0.02 | 0.12 ± 0.06 | 0.12 ± 0.05 | 0.23 ± 0.03 | 0.22 ± 0.01 |
| Direct bil (mg/dL) | 0.03–0.07 | 0.08 ± 0.02 | 0.08 ± 0.01 | 0.09 ± 0.03 | 0.01 ± 0.03 | 0.09 ± 0.02 | 0.08 ± 0.01 |
| Uric acid (mg/dL) | 1.4–3.7 | 4.2 ± 0.6 | 3.5 ± 1.1 | 3.9 ± 0.7 | 4.8 ± 1.0 | 5.0 ± 2.0 | 4.5 ± 1.8 |
| Sodium (mmol/L) | 140–150 | 143 ± 2 | 141 ± 1 | 143 ± 1 | 142 ± 1 | 143 ± 1 | 144 ± 1 |
| Potassium (mmol/L) | 4–5.9 | 5.3 ± 0.7 | 5.9 ± 0.5 | 5.5 ± 0.4 | 5.6 ± 0.5 | 4.9 ± 0.2 | 5.2 ± 0.3 |
| Chloride (mmol/L) | 96–107 | 104 ± 2 | 105 ± 1 | 105 ± 1 | 105 ± 1 | 102 ± 2 | 104 ± 1 |
| TCO2 (mmol/L) | 13–27.1 | 20.6 ± 1.5 | 22.6 ± 1.3 | 22.4 ± 1.6 | 22.9 ± 2.7 | 22.6 ± 2.5 | 19.5 ± 1.5 |
| Male | |||||||
| Glucose (mg/dL) | 85–132 | 214 ± 30 | 227 ± 44 | 290 ± 39 | 211 ± 45 | 176 ± 38 | 164 ± 23 |
| Cholesterol (mg/dL) | 40–130 | 66 ± 9 | 73 ± 20 | 64 ± 15 | 61 ± 9 | 73 ± 6 | 72 ± 10 |
| Triglyceride (mg/dL) | 80–190 | 40 ± 18 | 75 ± 24 | 47 ± 26 | 38 ± 12 | 75 ± 45 | 48 ± 23 |
| BUN (mg/dL) | 10–21 | 19 ± 2 | 18 ± 2 | 19 ± 2 | 17 ± 3 | 19 ± 3 | 17 ± 1 |
| Creatinine (mg/dL) | 0.5–1.0 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.0 | 0.8 ± 0.1 | 0.7 ± 0.0 # |
| Total protein (g/dL) | 6.3–8.6 | 6.7 ± 0.2 | 6.7 ± 0.2 | 6.9 ± 0.2 | 6.6 ± 0.1 | 6.8 ± 0.3 | 6.5 ± 0.1 |
| Albumin (g/dL) | 3.3–4.9 | 3.7 ± 0.1 | 3.6 ± 0.1 | 3.7 ± 0.1 | 3.6 ± 0.1 | 3.6 ± 0.1 | 3.5 ± 0.1 |
| AST (U/L) | 39–92 | 114 ± 14 | 108 ± 13 | 114 ± 22 | 100 ± 19 | 116 ± 32 | 95 ± 14 |
| ALT (U/L) | 17–50 | 30 ± 5 | 33 ± 4 | 40 ± 10 | 31 ± 6 | 31 ± 5 | 26 ± 3 |
| ALP (U/L) | 39–216 | 103 ± 19 | 93 ± 4 | 96 ± 10 | 87 ± 23 | 75 ± 18 | 66 ± 16 |
| Total bil (mg/dL) | 0.05–0.17 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.05 ± 0.02 | 0.06 ± 0.01 | 0.08 ± 0.01 | 0.06 ± 0.02 |
| Direct bil (mg/dL) | 0.03–0.07 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.04 ± 0.01 |
| Uric acid (mg/dL) | 1.4–3.7 | 5.3 ± 1.9 | 5.1 ± 1.4 | 7.3 ± 1.7 | 5.7 ± 0.8 | 2.8 ± 0.7 | 1.9 ± 0.6 |
| Sodium (mmol/L) | 140–150 | 145 ± 2 | 143 ± 4 | 144 ± 3 | 144 ± 1 | 146 ± 1 | 144 ± 1 # |
| Potassium (mmol/L) | 4–5.9 | 5.7 ± 0.5 | 6.1 ± 0.8 | 6.7 ± 0.7 | 7.1 ± 0.7 * | 5.2 ± 0.7 | 4.9 ± 0.6 |
| Chloride (mmol/L) | 96–107 | 100 ± 1 | 100 ± 3 | 101± 3 | 101 ± 2 | 101 ± 1 | 101 ± 1 |
| TCO2 (mmol/L) | 13–27.1 | 25.8 ± 2.0 | 27.1 ± 1.6 | 25.6 ± 2.1 | 25.6 ± 1.6 | 25.0 ± 1.1 | 27.4 ± 1.6 # |
| Fe-TA NPs Treatment (mg/kg bw) | Body Weight Gain (%) | Total Number of (Per 1000 Hepatocytes) | Mitotic Index | ||
|---|---|---|---|---|---|
| MN | MNH | BH | |||
| 5% Tween 80 | 30.8 ± 4.6 | 0.29 ± 0.39 | 0.29 ± 0.39 | 16.43 ± 2.82 | 0.72 ± 0.12 |
| Fe-TA NPs 55 mg/kg bw (Single dose) | 29.1 ± 4.8 | 0.68 ± 0.72 | 0.68 ± 0.72 | 18.60 ± 1.95 | 0.93 ± 0.12 |
| Fe-TA NPs 5.5 mg/kg bw (Single dose) | 32.4 ± 6.9 | 1.00 ± 0.93 | 1.00 ± 0.93 | 13.80 ± 0.84 | 0.79 ± 0.10 |
| Fe-TA NPs 5.5 mg/kg bw (Repeated doses) | 29.4 ± 4.2 | 0.30 ± 0.45 | 0.30 ± 0.45 | 16.00 ± 1.58 | 0.78 ± 0.13 |
| Parameters | Fe-TA NPs Treatment (mg/kg bw) | |||
|---|---|---|---|---|
| 0 | 0.55 | 5.5 | 17.5 | |
| Final Body Weight (g) | 495 ± 33 | 463 ± 19 | 466 ± 30 | 478 ± 49 |
| Absolute Organ weight (g) | ||||
| Liver | 15.77 ± 1.96 | 14.00 ± 1.86 | 14.76 ± 2.67 | 17.27 ± 2.28 |
| Spleen | 0.88 ± 0.06 | 0.82 ± 0.13 | 0.77 ± 0.06 | 0.80 ± 0.17 |
| Kidneys | 3.40 ± 0.35 | 3.25 ± 0.23 | 3.39 ± 0.14 | 3.40 ± 0.74 |
| Serum ALT levels (U/L) | 41.7 ± 14.8 | 37.5 ± 8.6 | 32.4 ± 6.0 | 39.0 ± 14.5 |
| Number of GST-P positive foci/Liver area (cm2) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Area of GST-P positive foci (mm2)/Liver area (cm2) | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Number of apoptotic cells/Liver area (mm2) | 5.27 ± 4.50 | 6.19 ± 1.48 | 6.17 ± 3.43 | 5.14 ± 1.01 |
| Number of PCNA-positive cells/Liver area (mm2) | 8.31 ± 2.09 | 7.66 ± 1.87 | 8.95 ± 2.53 | 7.15 ± 2.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hlaing, C.B.; Chariyakornkul, A.; Pilapong, C.; Punvittayagul, C.; Srichairatanakool, S.; Wongpoomchai, R. Assessment of Systemic Toxicity, Genotoxicity, and Early Phase Hepatocarcinogenicity of Iron (III)-Tannic Acid Nanoparticles in Rats. Nanomaterials 2022, 12, 1040. https://doi.org/10.3390/nano12071040
Hlaing CB, Chariyakornkul A, Pilapong C, Punvittayagul C, Srichairatanakool S, Wongpoomchai R. Assessment of Systemic Toxicity, Genotoxicity, and Early Phase Hepatocarcinogenicity of Iron (III)-Tannic Acid Nanoparticles in Rats. Nanomaterials. 2022; 12(7):1040. https://doi.org/10.3390/nano12071040
Chicago/Turabian StyleHlaing, Chi Be, Arpamas Chariyakornkul, Chalermchai Pilapong, Charatda Punvittayagul, Somdet Srichairatanakool, and Rawiwan Wongpoomchai. 2022. "Assessment of Systemic Toxicity, Genotoxicity, and Early Phase Hepatocarcinogenicity of Iron (III)-Tannic Acid Nanoparticles in Rats" Nanomaterials 12, no. 7: 1040. https://doi.org/10.3390/nano12071040
APA StyleHlaing, C. B., Chariyakornkul, A., Pilapong, C., Punvittayagul, C., Srichairatanakool, S., & Wongpoomchai, R. (2022). Assessment of Systemic Toxicity, Genotoxicity, and Early Phase Hepatocarcinogenicity of Iron (III)-Tannic Acid Nanoparticles in Rats. Nanomaterials, 12(7), 1040. https://doi.org/10.3390/nano12071040