CTAB Enhanced Room-Temperature Detection of NO2 Based on MoS2-Reduced Graphene Oxide Nanohybrid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Sample Preparation
2.3. Characterization of Materials
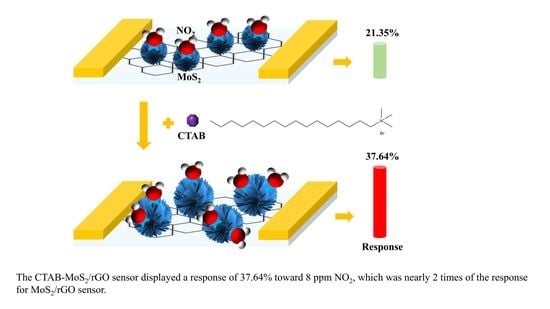
2.4. Fabrication of CTAB-MoS2/rGO Sensor
2.5. Measurement of Gas-Sensing Properties
3. Results
3.1. Morphological and Structural Characterization
3.2. Gas-Sensing Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rani, S.; Kumar, M.; Singh, Y.; Tomar, M.; Sharma, A.; Gupta, V.; Singh, V.N. NO2 Gas Sensor Based on SnSe/SnSe2 pn Hetrojunction. J. Nanosci. Nanotechnol. 2021, 21, 4779–4785. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Shen, Y.; Maboudian, R.; Carraro, C.; Han, C.; Liu, W.; Wei, D. Facile synthesis of ZnO-SnO2 hetero-structured nanowires for high-performance NO2 sensing application. Sens. Actuators B 2021, 333, 129613. [Google Scholar] [CrossRef]
- Zhu, Z.; Lin, S.J.; Wu, C.H.; Wu, R.J. Synthesis of TiO2 nanowires for rapid NO2 detection. Sens. Actuators A 2018, 272, 288–294. [Google Scholar] [CrossRef]
- Wang, X.D.; Wolfbeis, O.S. Fiber-optic chemical sensors and biosensors (2013–2015). Anal. Chem. 2016, 88, 203–227. [Google Scholar] [CrossRef]
- Chang, S.C.; Stetter, J.R. Electrochemical NO2 gas sensors: Model and mechanism for the electroreduction of NO2. Electroanalysis 1990, 2, 359–365. [Google Scholar] [CrossRef]
- Yang, B.; Myung, N.V.; Tran, T.T. 1D metal oxide semiconductor materials for chemiresistive gas sensors: A review. Adv. Electron. Mater. 2021, 7, 2100271. [Google Scholar] [CrossRef]
- Nasiri, N.; Clarke, C. Nanostructured chemiresistive gas sensors for medical applications. Sensors 2019, 19, 462. [Google Scholar] [CrossRef] [Green Version]
- Meng, F.L.; Guo, Z.; Huang, X.J. Graphene-based hybrids for chemiresistive gas sensors. TrAC Trends Anal. Chem. 2015, 68, 37–47. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, S.; Fei, T.; Liu, S.; Zhang, T. Construction of ZnO/SnO2 heterostructure on reduced graphene oxide for enhanced nitrogen dioxide sensitive performances at room temperature. ACS Sens. 2019, 4, 2048–2057. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Zhang, H.; Fei, T.; Zhang, T. Enhancing NO2 gas sensing performances at room temperature based on reduced graphene oxide-ZnO nanoparticles hybrids. Sens. Actuators B 2014, 202, 272–278. [Google Scholar] [CrossRef]
- Na, C.W.; Kim, J.H.; Kim, H.J.; Woo, H.S.; Gupta, A.; Kim, H.K.; Lee, J.H. Highly selective and sensitive detection of NO2 using rGO-In2O3 structure on flexible substrate at low temperature. Sens. Actuators B 2018, 255, 1671–1679. [Google Scholar]
- Wan, P.; Yang, W.; Wang, X.; Hu, J.; Zhang, H. Reduced graphene oxide modified with hierarchical flower-like In(OH)3 for NO2 room-temperature sensing. Sens. Actuators B 2015, 214, 36–42. [Google Scholar] [CrossRef]
- Yang, W.; Gan, L.; Li, H.; Zhai, T. Two-dimensional layered nanomaterials for gas-sensing applications. Inorg. Chem. Front. 2016, 3, 433–451. [Google Scholar]
- Late, D.J.; Huang, Y.K.; Liu, B.; Acharya, J.; Shirodkar, S.N.; Luo, J.; Yan, A.; Charles, D.; Waghmare, U.V.; Dravid, V.P.; et al. Sensing behavior of atomically thin-layered MoS2 transistors. ACS Nano 2013, 7, 4879–4891. [Google Scholar] [PubMed]
- Perkins, F.K.; Friedman, A.L.; Cobas, E.; Campbell, P.M.; Jernigan, G.G.; Jonker, B.T. Chemical vapor sensing with monolayer MoS2. Nano Lett. 2013, 13, 668–673. [Google Scholar] [CrossRef]
- Kumar, R.; Goel, N.; Kumar, M. UV-activated MoS2 based fast and reversible NO2 sensor at room temperature. ACS Sens. 2017, 2, 1744–1752. [Google Scholar] [CrossRef]
- Wang, S.; Chen, W.; Li, J.; Song, Z.; Zhang, H.; Zeng, W. Low working temperature of ZnO-MoS2 nanocomposites for delaying aging with good acetylene gas-sensing properties. Nanomaterials 2020, 10, 1902. [Google Scholar] [CrossRef]
- Lim, N.; Lee, J.S.; Byun, Y.T. Negatively-doped single-walled carbon nanotubes decorated with carbon dots for highly selective NO2 detection. Nanomaterials 2020, 10, 2509. [Google Scholar] [CrossRef]
- Zeng, Y.; Lin, S.; Gu, D.; Li, X. Two-dimensional nanomaterials for gas sensing applications: The role of theoretical calculations. Nanomaterials 2018, 8, 851. [Google Scholar] [CrossRef] [Green Version]
- Seo, W.S.; Kim, D.K.; Han, J.H.; Park, K.B.; Ryu, S.C.; Min, N.K.; Kim, J.H. Functionalization of Molybdenum Disulfide via Plasma Treatment and 3-Mercaptopropionic Acid for Gas Sensors. Nanomaterials 2020, 10, 1860. [Google Scholar] [CrossRef]
- Zhao, Z.; Bai, Y.; Ning, W.; Fan, J.; Gu, Z.; Chang, H.; Yin, S. Effect of surfactants on the performance of 3D morphology W18O49 by solvothermal synthesis. Appl. Surf. Sci. 2019, 471, 537–544. [Google Scholar] [CrossRef]
- Nagyné-Kovács, T.; Studnicka, L.; Lukács, I.E.; László, K.; Pasierb, P.; Szilágyi, I.M.; Pokol, G. Hydrothermal synthesis and gas sensing of monoclinic MoO3 nanosheets. Nanomaterials 2020, 10, 891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, W.; Li, Y. Hydrothermal synthesis and controlled growth of hierarchical 3D flower-like MoS2 nanospheres assisted with CTAB and their NO2 gas sensing properties. Appl. Surf. Sci. 2018, 455, 276–282. [Google Scholar] [CrossRef]
- Hongtao, L.; Zichen, X.; Lina, Z.; Zhiqiang, Z.; Li, X. The effects of different surfactants on the morphologies and electrochemical properties of MoS2/reduce graphene oxide composites. Chem. Phys. Lett. 2019, 716, 6–10. [Google Scholar] [CrossRef]
- Wu, J.; Sun, Y.M.; Wu, Z.; Li, X.; Wang, N.; Tao, K.; Wang, G.P. Carbon nanocoil-based fast-response and flexible humidity sensor for multifunctional applications. ACS Appl. Mater. Interfaces 2019, 11, 4242–4251. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Z.; Tao, K.; Liu, C.; Yang, B.R.; Xie, X.; Lu, X. Rapid-response, reversible and flexible humidity sensing platform using a hydrophobic and porous substrate. J. Mater. Chem. B 2019, 7, 2063–2073. [Google Scholar] [CrossRef]
- Shi, J.; Yu, P.; Liu, F.; He, P.; Wang, R.; Qin, L.; Zhou, J.; Sui, X.; Zhang, S.; Zhang, Y.; et al. 3R MoS2 with broken inversion symmetry: A promising ultrathin nonlinear optical device. Adv. Mater. 2017, 29, 1701486. [Google Scholar] [CrossRef]
- Chen, Y.; Song, B.; Tang, X.; Lu, L.; Xue, J. Ultrasmall Fe3O4 nanoparticle/MoS2 nanosheet composites with superior performances for lithium ion batteries. Small 2014, 10, 1536–1543. [Google Scholar] [CrossRef]
- Chang, K.; Mei, Z.; Wang, T.; Kang, Q.; Ouyang, S.; Ye, J. MoS2/graphene cocatalyst for efficient photocatalytic H2 evolution under visible light irradiation. ACS Nano 2014, 8, 7078–7087. [Google Scholar] [CrossRef]
- Chen, T.; Yan, W.; Xu, J.; Li, J.; Zhang, G.; Ho, D. Highly sensitive and selective NO2 sensor based on 3D MoS2/rGO composites prepared by a low temperature self-assembly method. J. Alloys Compd. 2019, 793, 541–551. [Google Scholar] [CrossRef]
- Cho, B.; Yoon, J.; Lim, S.K.; Kim, A.R.; Kim, D.H.; Park, S.G.; Kwon, J.D.; Lee, Y.J.; Lee, K.H.; Lee, B.H.; et al. Chemical sensing of 2D graphene/MoS2 heterostructure device. ACS Appl. Mater. Interfaces 2015, 7, 16775–16780. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.C.; Chen, D.; Miao, J.; Lin, S.; Yu, Z.; Han, Y.; Yang, Z.; Zhi, X.; Cui, D.X.; An, Z. Ag-Modified 3D Reduced Graphene Oxide Aerogel-Based Sensor with an Embedded Microheater for a Fast Response and High-Sensitive Detection of NO2. ACS Appl. Mater. Interfaces 2020, 12, 25243–25252. [Google Scholar] [CrossRef]
- Long, H.; Harley-Trochimczyk, A.; Pham, T.; Tang, Z.; Shi, T.; Zettl, A.; Carraro, C.; Marcus, A.W.; Maboudian, R. High surface area MoS2/graphene hybrid aerogel for ultrasensitive NO2 detection. Adv. Funct. Mater. 2016, 26, 5158–5165. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Yin, Z.; Du, Y.; Huang, X.; Zeng, Z.; Fan, Z.; Liu, H.; Wang, J.Y.; Zhang, H. Synthesis of few-layer MoS2 nanosheet-coated TiO2 nanobelt heterostructures for enhanced photocatalytic activities. Small 2013, 9, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Wang, Y.; Yu, L.; Wang, H.; Zhu, Y.; Li, C. Ag nanoparticles-modified Fe2O3@ MoS2 core-shell micro/nanocomposites for high-performance NO2 gas detection at low temperature. J. Alloys Compd. 2020, 829, 154471. [Google Scholar] [CrossRef]
- Donarelli, M.; Bisti, F.; Perrozzi, F.; Ottaviano, L. Tunable sulfur desorption in exfoliated MoS2 by means of thermal annealing in ultra-high vacuum. Chem. Phys. Lett. 2013, 588, 198–202. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Ye, Q.; Cinke, M.; Han, J.; Meyyappan, M. Carbon nanotube sensors for gas and organic vapor detection. Nano Lett. 2003, 3, 929–933. [Google Scholar] [CrossRef]
- Dai, Z.; Lee, C.S.; Tian, Y.; Kim, I.D.; Lee, J.H. Highly reversible switching from P-to N-type NO2 sensing in a monolayer Fe2O3 inverse opal film and the associated P–N transition phase diagram. J. Mater. Chem. A 2015, 3, 3372–3381. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, G.; Cheng, M.; Gao, Y.; Zhao, L.; Li, S.; Liu, F.M.; Yan, X.; Zhang, T.; Sun, P.; et al. The preparation of reduced graphene oxide-encapsulated α-Fe2O3 hybrid and its outstanding NO2 gas sensing properties at room temperature. Sens. Actuators B 2018, 261, 252–263. [Google Scholar] [CrossRef]
- Yao, F.; Duong, D.L.; Lim, S.C.; Yang, S.B.; Hwang, H.R.; Yu, W.J.; Lee, H.; Lee, Y.H. Humidity-assisted selective reactivity between NO2 and SO2 gas on carbon nanotubes. J. Mater. Chem. 2011, 21, 4502–4508. [Google Scholar] [CrossRef]
- Deokar, G.; Vancsó, P.; Arenal, R.; Ravaux, F.; Casanova-Cháfer, J.; Llobet, E.; Anna, M.; Densi, V.; Struzzi, C.; Lambin, P.; et al. MoS2–carbon nanotube hybrid material growth and gas sensing. Adv. Mater. Interfaces 2017, 4, 1700801. [Google Scholar] [CrossRef]
- Kim, Y.; Kwon, K.C.; Kang, S.; Kim, C.; Kim, T.H.; Hong, S.P.; Park, S.Y.; Suh, J.M.; Choi, M.J.; Han, S.; et al. Two-dimensional NbS2 gas sensors for selective and reversible NO2 detection at room temperature. ACS Sens. 2019, 4, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Wen, Z.; Huang, X.; Chang, J.; Chen, J. Stabilizing MoS2 nanosheets through SnO2 nanocrystal decoration for high-performance gas sensing in air. Small 2015, 11, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cheng, M.; Liu, G.; Gao, Y.; Zhao, L.; Li, S.; Wang, Y.P.; Liu, F.M.; Liang, X.S.; Zhang, T.; et al. Room temperature NO2 gas sensor based on porous Co3O4 slices/reduced graphene oxide hybrid. Sens. Actuators B 2018, 263, 387–399. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Z.; Ding, H.; Wei, Y.; Huang, W.; Yang, X.; Li, Z.Y.; Qiu, L.; Wang, X. Flexible, 3D SnS2/Reduced graphene oxide heterostructured NO2 sensor. Sens. Actuators B 2020, 305, 127445. [Google Scholar] [CrossRef]
- Li, W.; Chen, R.; Qi, W.; Cai, L.; Sun, Y.; Sun, M.; Li, C.; Yang, X.K.; Xiang, L.; Xie, D.; et al. Reduced graphene oxide/mesoporous ZnO NSs hybrid fibers for flexible, stretchable, twisted, and wearable NO2 E-textile gas sensor. ACS Sens. 2019, 4, 2809–2818. [Google Scholar] [CrossRef]
- Han, Y.; Huang, D.; Ma, Y.; He, G.; Hu, J.; Zhang, J.; Hu, N.T.; Su, Y.J.; Zhou, Z.H.; Zhang, Y.F.; et al. Design of hetero-nanostructures on MoS2 nanosheets to boost NO2 room-temperature sensing. ACS Appl. Mater. Interfaces 2018, 10, 22640–22649. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, D.; Chen, H. MOF-derived indium oxide hollow microtubes/MoS2 nanoparticles for NO2 gas sensing. Sens. Actuators B 2019, 300, 127037. [Google Scholar] [CrossRef]
- Kim, D.H.; Jung, J.W.; Choi, S.J.; Jang, J.S.; Koo, W.T.; Kim, I.D. Pt nanoparticles functionalized tungsten oxynitride hybrid chemiresistor: Low-temperature NO2 sensing. Sens. Actuators B 2018, 273, 1269–1277. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, G.; Zhu, X.; Guo, Y. Ultrasensitive NO2 gas sensing based on rGO/MoS2 nanocomposite film at low temperature. Sens. Actuators B 2017, 251, 280–290. [Google Scholar] [CrossRef]
- Mao, S.; Yu, K.; Cui, S.; Bo, Z.; Lu, G.; Chen, J. A new reducing agent to prepare single-layer, high-quality reduced graphene oxide for device applications. Nanoscale 2011, 3, 2849–2853. [Google Scholar] [CrossRef] [PubMed]









| Materials | Temperature (°C) | Concentration (ppm) | Response | LOD (ppb) | References |
|---|---|---|---|---|---|
| MoS2/CNT | RT | 25 | 5% a | 25 | [41] |
| ZnO-rGO | RT | 5 | 25.6% a | [9] | |
| NbS2 | RT | 5 | 18% a | 241.02 | [42] |
| MoS2/SnO2 | RT | 5 | 5% a | 500 | [43] |
| MoS2/rGO | RT | 5 | 14.28% a | 50 | [32] |
| rGO/Co3O4 | RT | 5 | 26.8% a | 50 | [44] |
| SnS2/rGO | RT | 8 | 49.8% b | 8.7 | [45] |
| rGO/ZnO | RT | 15 | 45% a | [46] | |
| MoS2/ZnO | RT | 5 | 3050% c | 50 | [47] |
| In2O3/MoS2 | RT | 10 | 50 d | 8.8 | [48] |
| CTAB-MoS2/rGO | RT | 8 | 37.64% a | 26.55 | Our work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Li, H.; Qian, R.; Zhuo, S.; Ju, P.; Chen, Q. CTAB Enhanced Room-Temperature Detection of NO2 Based on MoS2-Reduced Graphene Oxide Nanohybrid. Nanomaterials 2022, 12, 1300. https://doi.org/10.3390/nano12081300
Li W, Li H, Qian R, Zhuo S, Ju P, Chen Q. CTAB Enhanced Room-Temperature Detection of NO2 Based on MoS2-Reduced Graphene Oxide Nanohybrid. Nanomaterials. 2022; 12(8):1300. https://doi.org/10.3390/nano12081300
Chicago/Turabian StyleLi, Wenbo, Hao Li, Rong Qian, Shangjun Zhuo, Pengfei Ju, and Qiao Chen. 2022. "CTAB Enhanced Room-Temperature Detection of NO2 Based on MoS2-Reduced Graphene Oxide Nanohybrid" Nanomaterials 12, no. 8: 1300. https://doi.org/10.3390/nano12081300
APA StyleLi, W., Li, H., Qian, R., Zhuo, S., Ju, P., & Chen, Q. (2022). CTAB Enhanced Room-Temperature Detection of NO2 Based on MoS2-Reduced Graphene Oxide Nanohybrid. Nanomaterials, 12(8), 1300. https://doi.org/10.3390/nano12081300