Visualization and Comprehension of Electronic and Topographic Contrasts on Cooperatively Switched Diarylethene-Bridged Ditopic Ligand
Abstract
:1. Introduction
2. Materials and Methods
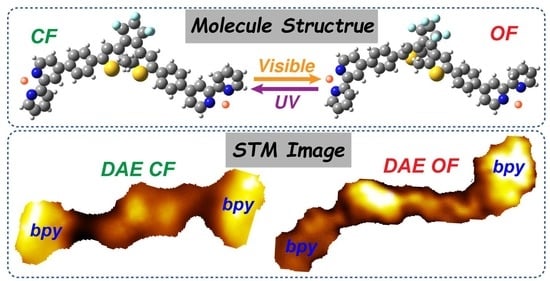
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Browne, W.R.; Feringa, B.L. Light switching of molecules on surfaces. Annu. Rev. Phys. Chem. 2009, 60, 407–428. [Google Scholar] [CrossRef]
- Kobatake, S.; Irie, M. Photochromism. Annu. Rep. Prog. Chem. Sect. C Phys. Chem. 2003, 99, 277–313. [Google Scholar] [CrossRef]
- Balzani, V.; Credi, A. Artificial molecular-level machines. Chem. Rec. 2001, 1, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Browne, W.W.R.; Feringa, B.L.B. Making molecular machines work. Nat. Nanotechnol. 2006, 1, 25–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Li, Q. Photochromism into nanosystems: Towards lighting up the future nanoworld. Chem. Soc. Rev. 2018, 47, 1044–1097. [Google Scholar] [CrossRef] [PubMed]
- Dela Cruz Calupitan, J.P.; Galangau, O.; Nakashima, T.; Kawai, T.; Rapenne, G. Photochromic Diarylethenes Designed for Surface Deposition: From Self-Assembled Monolayers to Single Molecules. Chempluschem 2019, 84, 564–577. [Google Scholar] [CrossRef]
- Irie, M.; Fukaminato, T.; Matsuda, K.; Kobatake, S. Photochromism of diarylethene molecules and crystals: Memories, switches, and actuators. Chem. Rev. 2014, 114, 12174–12277. [Google Scholar] [CrossRef]
- Abendroth, J.M.; Bushuyev, O.S.; Weiss, P.S.; Barrett, C.J. Controlling Motion at the Nanoscale: Rise of the Molecular Machines. ACS Nano 2015, 9, 7746–7768. [Google Scholar] [CrossRef]
- Ragazzon, G.; Baroncini, M.; Silvi, S.; Venturi, M.; Credi, A. Light-powered autonomous and directional molecular motion of a dissipative self-assembling system. Nat. Nanotechnol. 2015, 10, 70–75. [Google Scholar] [CrossRef]
- Nacci, C.; Baroncini, M.; Credi, A.; Grill, L. Reversible Photoswitching and Isomer-Dependent Diffusion of Single Azobenzene Tetramers on a Metal Surface. Angew. Chem. Int. Ed. 2018, 57, 15034–15039. [Google Scholar] [CrossRef] [Green Version]
- Saywell, A.; Bakker, A.; Mielke, J.; Kumagai, T.; Wolf, M.; García-López, V.; Chiang, P.-T.; Tour, J.M.; Grill, L. Light-Induced Translation of Motorized Molecules on a Surface. ACS Nano 2016, 10, 10945–10952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foy, J.T.; Li, Q.; Goujon, A.; Colard-Itté, J.-R.; Fuks, G.; Moulin, E.; Schiffmann, O.; Dattler, D.; Funeriu, D.P.; Giuseppone, N. Dual-light control of nanomachines that integrate motor and modulator subunits. Nat. Nanotechnol. 2017, 12, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; You, M.; Wu, C.; Han, D.; Öçsoy, I.; Chen, T.; Chen, Z.; Tan, W. Reversible Phase Transfer of Nanoparticles Based on Photoswitchable Host–Guest Chemistry. ACS Nano 2014, 8, 2555–2561. [Google Scholar] [CrossRef]
- Zhao, H.; Sen, S.; Udayabhaskararao, T.; Sawczyk, M.; Kučanda, K.; Manna, D.; Kundu, P.K.; Lee, J.-W.; Král, P.; Klajn, R. Reversible trapping and reaction acceleration within dynamically self-assembling nanoflasks. Nat. Nanotechnol. 2016, 11, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.K.; Samanta, D.; Leizrowice, R.; Margulis, B.; Zhao, H.; Börner, M.; Udayabhaskararao, T.; Manna, D.; Klajn, R. Light-controlled self-assembly of non-photoresponsive nanoparticles. Nat. Chem. 2015, 7, 646–652. [Google Scholar] [CrossRef]
- Díaz, S.A.; Giordano, L.; Azcárate, J.C.; Jovin, T.M.; Jares-Erijman, E.A. Quantum Dots as Templates for Self-Assembly of Photoswitchable Polymers: Small, Dual-Color Nanoparticles Capable of Facile Photomodulation. J. Am. Chem. Soc. 2013, 135, 3208–3217. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Fukaminato, T.; Placial, J.-P.; Onodera, T.; Suzuki, R.; Oikawa, H.; Brosseau, A.; Brisset, F.; Pansu, R.; Nakatani, K.; et al. Giant Amplification of Photoswitching by a Few Photons in Fluorescent Photochromic Organic Nanoparticles. Angew. Chemie Int. Ed. 2016, 55, 3662–3666. [Google Scholar] [CrossRef]
- Katsonis, N.; Kudernac, T.; Walko, M.; Van Der Molen, S.J.; Van Wees, B.J.; Feringa, B.L. Reversible conductance switching of single diarylethenes on a gold surface. Adv. Mater. 2006, 18, 1397–1400. [Google Scholar] [CrossRef]
- Jia, C.; Migliore, A.; Xin, N.; Huang, S.; Wang, J.; Yang, Q.; Wang, S.; Chen, H.; Wang, D.; Feng, B.; et al. Covalently bonded single-molecule junctions with stable and reversible photoswitched conductivity. Science 2016, 352, 1443–1445. [Google Scholar] [CrossRef]
- Thomas, L.; Arbouch, I.; Guérin, D.; Wallart, X.; van Dyck, C.; Mélin, T.; Cornil, J.; Vuillaume, D.; Lenfant, S. Conductance switching of azobenzene-based self-assembled monolayers on cobalt probed by UHV conductive-AFM. Nanoscale 2021, 13, 6977–6990. [Google Scholar] [CrossRef]
- Hnid, I.; Frath, D.; Lafolet, F.; Sun, X.; Lacroix, J.-C. Highly Efficient Photoswitch in Diarylethene-Based Molecular Junctions. J. Am. Chem. Soc. 2020, 142, 7732–7736. [Google Scholar] [CrossRef]
- Hnid, I.; Liu, M.; Frath, D.; Bellynck, S.; Lafolet, F.; Sun, X.; Lacroix, J.-C. Unprecedented ON/OFF Ratios in Photoactive Diarylethene-Bisthienylbenzene Molecular Junctions. Nano Lett. 2021, 21, 7555–7560. [Google Scholar] [CrossRef]
- Hnid, I.; Grempka, A.; Khettabi, A.; Sun, X.; Lacroix, J.C.; Lafolet, F.; Cobo, S. Combining Photomodulation and Rectification in Coordination Molecular Wires Based on Dithienylethene Molecular Junctions. J. Phys. Chem. C 2020, 124, 26304–26309. [Google Scholar] [CrossRef]
- Leydecker, T.; Herder, M.; Pavlica, E.; Bratina, G.; Hecht, S.; Orgiu, E.; Samori, P. Flexible non-volatile optical memory thin-film transistor device with over 256 distinct levels based on an organic bicomponent blend. Nat. Nanotechnol. 2016, 11, 769–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Gemayel, M.; Börjesson, K.; Herder, M.; Duong, D.T.; Hutchison, J.A.; Ruzié, C.; Schweicher, G.; Salleo, A.; Geerts, Y.; Hecht, S.; et al. Optically switchable transistors by simple incorporation of photochromic systems into small-molecule semiconducting matrices. Nat. Commun. 2015, 6, 6330. [Google Scholar] [CrossRef]
- Orgiu, E.; Samorì, P. 25th anniversary article: Organic electronics marries photochromism: Generation of multifunctional interfaces, materials, and devices. Adv. Mater. 2014, 26, 1827–1845. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Michel, R.; He, B.; Chen, Y.-S.; Stalke, D.; John, M.; Clever, G.H. Light-Triggered Guest Uptake and Release by a Photochromic Coordination Cage. Angew. Chem. Int. Ed. 2013, 52, 1319–1323. [Google Scholar] [CrossRef]
- Yanai, N.; Uemura, T.; Inoue, M.; Matsuda, R.; Fukushima, T.; Tsujimoto, M.; Isoda, S.; Kitagawa, S. Guest-to-Host Transmission of Structural Changes for Stimuli-Responsive Adsorption Property. J. Am. Chem. Soc. 2012, 134, 4501–4504. [Google Scholar] [CrossRef]
- Huang, H.; Sato, H.; Aida, T. Crystalline Nanochannels with Pendant Azobenzene Groups: Steric or Polar Effects on Gas Adsorption and Diffusion? J. Am. Chem. Soc. 2017, 139, 8784–8787. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Y.; Yan, Y.; Huang, J. Smart Nanocarrier: Self-Assembly of Bacteria-like Vesicles with Photoswitchable Cilia. ACS Nano 2014, 8, 11341–11349. [Google Scholar] [CrossRef]
- Cai, Y.; Guo, Z.; Chen, J.; Li, W.; Zhong, L.; Gao, Y.; Jiang, L.; Chi, L.; Tian, H.; Zhu, W.-H. Enabling Light Work in Helical Self-Assembly for Dynamic Amplification of Chirality with Photoreversibility. J. Am. Chem. Soc. 2016, 138, 2219–2224. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; van Leeuwen, T.; Cheng, J.; Feringa, B.L. Dynamic control of chirality and self-assembly of double-stranded helicates with light. Nat. Chem. 2017, 9, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Mogaki, R.; Okuro, K.; Aida, T. Adhesive Photoswitch: Selective Photochemical Modulation of Enzymes under Physiological Conditions. J. Am. Chem. Soc. 2017, 139, 10072–10078. [Google Scholar] [CrossRef]
- Velema, W.A.; Szymanski, W.; Feringa, B.L. Photopharmacology: Beyond proof of principle. J. Am. Chem. Soc. 2014, 136, 2178–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irie, M.; Mohri, M. Thermally Irreversible Photochromic Systems. Reversible Photocyclization of Diarylethene Derivatives. J. Org. Chem. 1988, 53, 803–808. [Google Scholar] [CrossRef]
- Snegir, S.V.; Marchenko, A.A.; Yu, P.; Maurel, F.; Kapitanchuk, O.L.; Mazerat, S.; Lepeltier, M.; Léaustic, A.; Lacaze, E. STM Observation of Open- and Closed-Ring Forms of Functionalized Diarylethene Molecules Self-Assembled on a Au(111) Surface. J. Phys. Chem. Lett. 2011, 2, 2433–2436. [Google Scholar] [CrossRef]
- Jia, C.; Wang, J.; Yao, C.; Cao, Y.; Zhong, Y.; Liu, Z.; Liu, Z.; Guo, X. Conductance switching and mechanisms in single-molecule junctions. Angew. Chem. Int. Ed. 2013, 52, 8666–8670. [Google Scholar] [CrossRef]
- Hnid, I.; Sun, X.; Frath, D.; Lafolet, F.; Lacroix, J.C. Correction: Multi-functional switches of ditopic ligands with azobenzene central bridges at a molecular scale. Nanoscale 2020, 12, 1181. [Google Scholar] [CrossRef] [Green Version]
- Bonacchi, S.; El Garah, M.; Ciesielski, A.; Herder, M.; Conti, S.; Cecchini, M.; Hecht, S.; Samorì, P. Surface-induced selection during in situ photoswitching at the solid/liquid interface. Angew. Chem. Int. Ed. 2015, 54, 4865–4869. [Google Scholar] [CrossRef]
- Mielke, J.; Leyssner, F.; Koch, M.; Meyer, S.; Luo, Y.; Selvanathan, S.; Haag, R.; Tegeder, P.; Grill, L. Imine Derivatives on Au(111): Evidence for “Inverted” Thermal Isomerization. ACS Nano 2011, 5, 2090–2097. [Google Scholar] [CrossRef]
- Alemani, M.; Peters, M.V.; Hecht, S.; Rieder, K.H.; Moresco, F.; Grill, L. Electric field-induced isomerization of azobenzene by STM. J. Am. Chem. Soc. 2006, 128, 14446–14447. [Google Scholar] [CrossRef] [PubMed]
- Comstock, M.J.; Levy, N.; Kirakosian, A.; Cho, J.; Lauterwasser, F.; Harvey, J.H.; Strubbe, D.A.; Fréchet, J.M.J.; Trauner, D.; Louie, S.G.; et al. Reversible photomechanical switching of individual engineered molecules at a metallic surface. Phys. Rev. Lett. 2007, 99, 038301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.L.; Zhong, J.Q.; Lin, J.D.; Hu, W.P.; Wu, K.; Xu, G.Q.; Wee, A.T.S.; Chen, W. Towards single molecule switches. Chem. Soc. Rev. 2015, 44, 2998–3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grim, P.C.M.; Vanoppen, P.; Rucker, M.; De Feyter, S.; Valiyaveettil, S.; Moessner, G.; Mullen, K.; De Schryver, F.C. Molecular organization of azobenzene derivatives at the liquid/graphite interface observed with scanning tunneling microscopy. J. Vac. Sci. Technol. B. 1997, 15, 1419–1424. [Google Scholar] [CrossRef]
- Tahara, K.; Inukai, K.; Adisoejoso, J.; Yamaga, H.; Balandina, T.; Blunt, M.O.; De Feyter, S.; Tobe, Y. Tailoring surface-confined nanopores with photoresponsive groups. Angew. Chem. Int. Ed. 2013, 52, 8373–8376. [Google Scholar] [CrossRef]
- El Garah, M.; Borré, E.; Ciesielski, A.; Dianat, A.; Gutierrez, R.; Cuniberti, G.; Bellemin-Laponnaz, S.; Mauro, M.; Samorì, P. Light-Induced Contraction/Expansion of 1D Photoswitchable Metallopolymer Monitored at the Solid–Liquid Interface. Small 2017, 13, 1701790. [Google Scholar] [CrossRef] [PubMed]
- Galanti, A.; Diez-Cabanes, V.; Santoro, J.; Valášek, M.; Minoia, A.; Mayor, M.; Cornil, J.; Samorì, P. Electronic Decoupling in C3-Symmetrical Light-Responsive Tris(Azobenzene) Scaffolds: Self-Assembly and Multiphotochromism. J. Am. Chem. Soc. 2018, 140, 16062–16070. [Google Scholar] [CrossRef] [Green Version]
- Pace, G.; Ferri, V.; Grave, C.; Elbing, M.; von Hanisch, C.; Zharnikov, M.; Mayor, M.; Rampi, M.A.; Samori, P. Cooperative light-induced molecular movements of highly ordered azobenzene self-assembled monolayers. Proc. Natl. Acad. Sci. USA 2007, 104, 9937–9942. [Google Scholar] [CrossRef] [Green Version]
- Frath, D.; Yokoyama, S.; Hirose, T.; Matsuda, K. Photoresponsive supramolecular self-assemblies at the liquid/solid interface. J. Photochem. Photobiol. C Photochem. Rev. 2018, 34, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Snegir, S.V.; Yu, P.; Maurel, F.; Kapitanchuk, O.L.; Marchenko, A.A.; Lacaze, E. Switching at the Nanoscale: Light- and STM-Tip-Induced Switch of a Thiolated Diarylethene Self-Assembly on Au(111). Langmuir 2014, 30, 13556–13563. [Google Scholar] [CrossRef]
- Katsonis, N.; Minoia, A.; Kudernac, T.; Mutai, T.; Xu, H.; Uji-i, H.; Lazzaroni, R.; De Feyter, S.; Feringa, B.L. Locking of helicity and shape complementarity in diarylethene dimers on graphite. J. Am. Chem. Soc. 2008, 130, 386–387. [Google Scholar] [CrossRef] [PubMed]
- Peyrot, D.; Silly, F. On-Surface Synthesis of Two-Dimensional Covalent Organic Structures versus Halogen-Bonded Self-Assembly: Competing Formation of Organic Nanoarchitectures. ACS Nano 2016, 10, 5490–5498. [Google Scholar] [CrossRef] [PubMed]
- Barth, J.V. Fresh perspectives for surface coordination chemistry. Surf. Sci. 2009, 603, 1533–1541. [Google Scholar] [CrossRef]
- Bischoff, F.; Seufert, K.; Auwärter, W.; Joshi, S.; Vijayaraghavan, S.; Écija, D.; Diller, K.; Papageorgiou, A.C.; Fischer, S.; Allegretti, F.; et al. How Surface Bonding and Repulsive Interactions Cause Phase Transformations: Ordering of a Prototype Macrocyclic Compound on Ag(111). ACS Nano 2013, 7, 3139–3149. [Google Scholar] [CrossRef] [PubMed]
- Reecht, G.; Heinrich, B.W.; Bulou, H.; Scheurer, F.; Limot, L.; Schull, G. Imaging isodensity contours of molecular states with STM. New J. Phys. 2017, 19, 113033. [Google Scholar] [CrossRef]
- Yang, P.; Li, D.; Repain, V.; Chacon, C.; Girard, Y.; Rousset, S.; Smogunov, A.; Dappe, Y.J.; Barreteau, C.; Lagoute, J. C60 as an Atom Trap to Capture Co Adatoms. J. Phys. Chem. C 2015, 119, 6873–6879. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wu, K.; Kröger, J.; Berndt, R.; KrögerKr, O. Review Article: Structures of phthalocyanine molecules on surfaces studied by STM. AIP Adv. 2012, 2, 41402. [Google Scholar] [CrossRef]
- Gross, L.; Moll, N.; Mohn, F.; Curioni, A.; Meyer, G.; Hanke, F.; Persson, M. High-Resolution Molecular Orbital Imaging Using a p-Wave STM Tip. Phys. Rev. Lett. 2011, 107, 086101. [Google Scholar] [CrossRef] [Green Version]
- Schwöbel, J.; Fu, Y.; Brede, J.; Dilullo, A.; Hoffmann, G.; Klyatskaya, S.; Ruben, M.; Wiesendanger, R. Real-space observation of spin-split molecular orbitals of adsorbed single-molecule magnets. Nat. Commun. 2012, 3, 953. [Google Scholar] [CrossRef] [Green Version]
- Jelinek, P. High resolution SPM imaging of organic molecules with functionalized tips. J. Phys. Condens. Matter 2017, 29, 343002. [Google Scholar] [CrossRef]
- Wang, Y.; Kröger, J.; Berndt, R.; Hofer, W. Structural and electronic properties of ultrathin tin-phthalocyanine films on Ag(111) at the single-molecule level. Angew. Chem. Int. Ed. 2009, 48, 1261–1265. [Google Scholar] [CrossRef]
- Silly, F. A robust method for processing scanning probe microscopy images and determining nanoobject position and dimensions. J. Microsc. 2009, 236, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [Green Version]
- Jansen, H.B.; Ros, P. Non-empirical molecular orbital calculations on the protonation of carbon monoxide. Chem. Phys. Lett. 1969, 3, 140–143. [Google Scholar] [CrossRef]
- Lui, B.; McLean, A.D. Accurate calculation of the attractive interaction of two ground state helium atoms. J. Chem. Phys. 1973, 59, 4557–4558. [Google Scholar]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Sun, X.; Lafolet, F.; Lemercier, G.; Maurel, F.; Lacroix, J.C. Molecular Isomerization and Multiscale Phase Transitions of a Ditopic Ligand on a Surface. J. Phys. Chem. C 2017, 121, 20925–20930. [Google Scholar] [CrossRef]
- Sun, X.; Frath, D.; Lafolet, F.; Lacroix, J.-C. Supramolecular Networks and Wires Dominated by Intermolecular BiEDOT Interactions. J. Phys. Chem. C 2018, 122, 22760–22766. [Google Scholar] [CrossRef]
- Sun, X.; Yao, X.; Lafolet, F.; Lemercier, G.; Lacroix, J.-C. One-Dimensional Double Wires and Two-Dimensional Mobile Grids: Cobalt/Bipyridine Coordination Networks at the Solid/Liquid Interface. J. Phys. Chem. Lett. 2019, 10, 4164–4169. [Google Scholar] [CrossRef]
- Scheil, K.; Gopakumar, T.G.; Bahrenburg, J.; Temps, F.; Maurer, R.J.; Reuter, K.; Berndt, R. Switching of an Azobenzene-Tripod Molecule on Ag(111). J. Phys. Chem. Lett. 2016, 7, 2080–2084. [Google Scholar] [CrossRef] [Green Version]
- Dulić, D.; van der Molen, S.J.; Kudernac, T.; Jonkman, H.T.; de Jong, J.J.D.; Bowden, T.N.; van Esch, J.; Feringa, B.L.; van Wees, B.J. One-way optoelectronic switching of photochromic molecules on gold. Phys. Rev. Lett. 2003, 91, 207402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kervella, Y.; Shilova, E.; Latil, S.; Jousselme, B.; Silly, F. S-Shaped Conformation of the Quaterthiophene Molecular Backbone in Two-Dimensional Bisterpyridine-Derivative Self-Assembled Nanoarchitecture. Langmuir 2015, 31, 13420–13425. [Google Scholar] [CrossRef] [PubMed]
- Velpula, G.; Teyssandier, J.; De Feyter, S.; Mali, K.S. Nanoscale Control over the Mixing Behavior of Surface-Confined Bicomponent Supramolecular Networks Using an Oriented External Electric Field. ACS Nano 2017, 11, 10903–10913. [Google Scholar] [CrossRef] [PubMed]
- Silly, F.; Ausset, S.; Sun, X. Coexisting Chiral Two-Dimensional Self-Assembled Structures of 1,2,3,4-Tetrahydronaphthalene Molecules: Porous Pinwheel Nanoarchitecture and Close-Packed Herringbone Arrangement. J. Phys. Chem. C 2017, 121, 15288–15293. [Google Scholar] [CrossRef] [Green Version]
- Chng, G.Y.Y.; Sun, X.; Cho, S.J.; Rajwar, D.; Grimsdale, A.C.; Fichou, D. Synthesis and 2D self-assembly at the liquid-solid interface of novel H-bonding linear p-conjugated oligomers terminated by uracil and melamine units. New J. Chem 2014, 38, 2407. [Google Scholar] [CrossRef]
- Miura, A.; Chen, Z.; Uji-I, H.; De Feyter, S.; Zdanowska, M.; Jonkheijm, P.; Schenning, A.P.H.J.; Meijer, E.W.; Würthner, F.; De Schryver, F.C. Bias-Dependent Visualization of Electron Donor (D) and Electron Acceptor (A) Moieties in a Chiral DAD Triad Molecule. J. Am. Chem. Soc. 2003, 125, 14968–14969. [Google Scholar] [CrossRef]
- Lei, S.; De Feyter, S. STM, STS and bias-dependent imaging on organic monolayers at the solid-liquid interface. Top. Curr. Chem. 2008, 285, 269–312. [Google Scholar]
- Wang, D.; Wang, Z.; Liu, W.; Zhong, S.; Wee, A.T.S. Real-space Imaging of the Multiple Halogen Bonds by Ultrahigh-resolution Scanning Probe Microscopy. ChemRxiv, 2021; in preprint. [Google Scholar]
- Goronzy, D.P.; Ebrahimi, M.; Rosei, F.; Arramel; Fang, Y.; De Feyter, S.; Tait, S.L.; Wang, C.; Beton, P.H.; Wee, A.T.S.; et al. Supramolecular assemblies on surfaces: Nanopatterning, functionality, and reactivity. ACS Nano 2018, 12, 7445–7481. [Google Scholar] [CrossRef]
- Cheng, Z.; Du, S.; Guo, W.; Gao, L.; Deng, Z.; Jiang, N.; Guo, H.; Tang, H.; Gao, H.J. Direct imaging of molecular orbitals of metal phthalocyanines on metal surfaces with an O2-functionalized tip of a scanning tunneling microscope. Nano Res. 2011, 4, 523–530. [Google Scholar] [CrossRef]
- Gimzewski, J.K.; Joachim, C. Nanoscale Science of Single Molecules Using Local Probes. Science 1999, 283, 1683–1688. [Google Scholar] [CrossRef] [PubMed]
- Maurel, F.; Perrier, A.; Jacquemin, D. An ab initio simulation of a dithienylethene/phenoxynaphthacenequinone photochromic hybrid. J. Photochem. Photobiol. A Chem. 2011, 218, 33–40. [Google Scholar] [CrossRef]
- Yokojima, S.; Kobayashi, T.; Shinoda, K.; Matsuda, K.; Higashiguchi, K.; Nakamura, S. π-Conjugation of Two Nitronyl Nitroxides-Attached Diarylethenes. J. Phys. Chem. B 2011, 115, 5685–5692. [Google Scholar] [CrossRef]
- Perrier, A.; Maurel, F.; Browne, W.R.; Jacquemin, D. Full ring closing in a diarylethene hexamer: Insights from theory. Chem. Commun. 2013, 49, 4247–4249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arai, R.; Uemura, S.; Irie, M.; Matsuda, K. Reversible photoinduced change in molecular ordering of diarylethene derivatives at a solution-HOPG interface. J. Am. Chem. Soc. 2008, 130, 9371–9379. [Google Scholar] [CrossRef]
- Yu, Y.J.; Zhao, Y.; Ryu, S.; Brus, L.E.; Kim, K.S.; Kim, P. Tuning the graphene work function by electric field effect. Nano Lett. 2009, 9, 3430–3434. [Google Scholar] [CrossRef] [Green Version]
- Diez Cabanes, V.; Van Dyck, C.; Osella, S.; Cornil, D.; Cornil, J. Challenges for Incorporating Optical Switchability in Organic-Based Electronic Devices. ACS Appl. Mater. Interfaces 2021, 13, 27737–27748. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hnid, I.; Guan, L.; Chatir, E.; Cobo, S.; Lafolet, F.; Maurel, F.; Lacroix, J.-C.; Sun, X. Visualization and Comprehension of Electronic and Topographic Contrasts on Cooperatively Switched Diarylethene-Bridged Ditopic Ligand. Nanomaterials 2022, 12, 1318. https://doi.org/10.3390/nano12081318
Hnid I, Guan L, Chatir E, Cobo S, Lafolet F, Maurel F, Lacroix J-C, Sun X. Visualization and Comprehension of Electronic and Topographic Contrasts on Cooperatively Switched Diarylethene-Bridged Ditopic Ligand. Nanomaterials. 2022; 12(8):1318. https://doi.org/10.3390/nano12081318
Chicago/Turabian StyleHnid, Imen, Lihao Guan, Elarbi Chatir, Saioa Cobo, Frédéric Lafolet, François Maurel, Jean-Christophe Lacroix, and Xiaonan Sun. 2022. "Visualization and Comprehension of Electronic and Topographic Contrasts on Cooperatively Switched Diarylethene-Bridged Ditopic Ligand" Nanomaterials 12, no. 8: 1318. https://doi.org/10.3390/nano12081318
APA StyleHnid, I., Guan, L., Chatir, E., Cobo, S., Lafolet, F., Maurel, F., Lacroix, J. -C., & Sun, X. (2022). Visualization and Comprehension of Electronic and Topographic Contrasts on Cooperatively Switched Diarylethene-Bridged Ditopic Ligand. Nanomaterials, 12(8), 1318. https://doi.org/10.3390/nano12081318