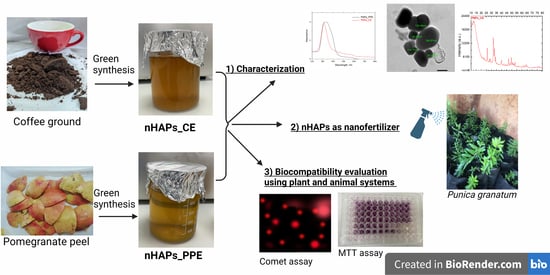
Green Synthesis of Phosphorous-Containing Hydroxyapatite Nanoparticles (nHAP) as a Novel Nano-Fertilizer: Preliminary Assessment on Pomegranate (Punica granatum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells and Chemicals
2.2. Plant Extracts Preparation and Green Synthesis of Phosphorous Nanoparticles
2.3. Characterization of Phosphorous Nanoparticles
2.3.1. UV–VIS Spectroscopy
2.3.2. Surface Morphology Using SEM, TEM, and XRD
2.3.3. Dynamic Light Scattering (DLS) Analysis
2.3.4. Fourier Transform Infrared Spectroscopy (FTIR)
2.4. Cytotoxicity MTT Assay and IC50 Determination
2.5. Plant Materials and nHAPs Applications
2.5.1. Experiment Design
2.5.2. Treatments
2.5.3. Determination of Photosynthetic Pigments
2.5.4. Determination of Total Soluble Carbohydrates
2.6. Metabolite Profiling Using GC/MS
2.7. Biocompatibility Evaluation Using Plant System
2.7.1. Cell Membrane Stability
2.7.2. DNA Fragmentation Test (Comet Assay)
3. Results
3.1. Characterization of Phosphorous Nanoparticles
3.2. Biochemical Analysis
3.2.1. Photosynthetic Pigments
3.2.2. Total Soluble Carbohydrates
3.3. Metabolite Profiling
3.4. Biocompatibility Assays
3.4.1. Testing on Mammalian Cells Using MTT Assay
3.4.2. Testing on Plant Cells
4. Discussion
4.1. nHAP Preparation and Characterization
4.2. Biochemical Analysis
4.2.1. Photosynthetic Pigments
4.2.2. Total Soluble Carbohydrates
4.3. Metabolite Profiling
4.4. Biocompatibility with the Biological Systems
4.4.1. MTT Assay with Mammalian Cells
4.4.2. Biocompatibility Assays of nHAPs with Punica granatum Plants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez-Merino, F.C.; Trejo-Téllez, L.I. The role of beneficial elements in triggering adaptive responses to environmental stressors and improving plant performance. In Biotic and Abiotic Stress Tolerance in Plants; Springe Nature: Singapore, 2018; pp. 137–172. [Google Scholar]
- UNEP. Emerging challenges-nanotechnology and the environment. In GEO Yearbook; United Nations Environment Programme Division of Early Warning and Assessment: Nairobi, Kenya, 2007; pp. 61–70. [Google Scholar]
- Akhtar, N.; IIyas, N.; Meraj, T.A.; Pour-Aboughadareh, A.; Sayyed, R.Z.; Mashwani, Z.U.R.; Poczai, P. Improvement of plant responses by Nanobiofertilizer: A step towards Sustinable Agriculture. Nanomaterials 2022, 12, 965. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Sabir, M.; Farooq, M.; Maqsood, M.A.; Ahmad, H.R.; Warraich, E.A. Phosphorus deficiency in plants: Responses, adaptive mechanisms and signaling. In Plant Signaling: Understanding the Molecular Crosstalk; Hakeem, K., Rehman, R., Tahir, I., Eds.; Springer: New Delhi, India, 2013; pp. 133–148. [Google Scholar]
- Simpson, R.J.; Oberson, A.; Culvenor, R.A.; Ryan, M.H.; Veneklaas, E.J.; Lambers, H.; Lynch, J.P.; Ryan, P.R.; Delhaize, E.; Smith, F.A.; et al. Strategies and agronomic interventions to improve the phosphorus-use efficiency of farming systems. Plant Soil 2011, 349, 89–120. [Google Scholar] [CrossRef]
- Smith, V.H.; Schindler, D.W. Eutrophication science: Where do we go from here? Trends Ecol. Evol. 2009, 24, 201–207. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Schröder, J.J.; Smith, A.L.; Cordell, D.; Rosemarin, A. Improved phosphorus use efficiency in agriculture: A keyrequirement for its sustainable use. Chemosphere 2011, 84, 822–831. [Google Scholar] [CrossRef]
- Watanabe, Y.; Yamada, H.; Ikoma, T.; Tanaka, J.; Stevens, G.W.; Komatsu, Y. Preparation of a zeolite NaP1/hydroxyapatite nanocomposite and study of its behavior as inorganic fertilizer. J. Chem. Technol. Biotechnol. 2014, 89, 963–968. [Google Scholar] [CrossRef]
- Kottegoda, N.; Munaweera, I.; Madusanka, N.; Karunaratne, V. A green slow-release fertilizer composition based on urea-modified hydroxyapatite nanoparticles encapsuled wood. Curr. Sci. 2011, 101, 73–78. [Google Scholar]
- Soliman, A.S.; Hassan, M.; Abo-Elella, F.; Hanafy Ahmed, A.H.; El-Feky, S.A. Effect of Nano and molecular phosphorous fertilizers on growth and chemical composition of Baobab (Adansonia digitata L.). J. Plant Sci. 2016, 11, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Nayak, A.K. Hydroxyapatite synthesis methodologies: An overview. Int. J. ChemTech Res. 2010, 2, 903–907. [Google Scholar]
- Saratale, R.G.; Karuppusamy, I.; Saratale, G.D.; Pugazhendhi, A.; Kumar, G.; Park, Y.; Ghodake, G.S.; Bharagava, R.N.; Banu, J.R.; Shin, H.S. A comprehensive review on green nanomaterials using biological systems: Recent perception and their future applications. Colloids Surf. B Biointerfaces 2018, 170, 20–35. [Google Scholar] [CrossRef]
- Alyamani, A.A.; Albukhaty, S.; Aloufi, S.; AlMalki, F.A.; Al-Karagoly, H.; Sulaiman, G.M. Green Fabrication of Zinc Oxide Nanoparticles Using Phlomis Leaf Extract: Characterization and In Vitro Evaluation of Cytotoxicity and Antibacterial Properties. Molecules 2021, 26, 6140. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, R.; MubarakAli, D.; Prabakar, D.; Muthukumar, H.; Thajuddin, N.; Kumar, S.S.; Pugazhendhi, A. An enhancement of antimicrobial efficacy of biogenic and ceftriaxone-conjugated silver nanoparticles: Green approach. Environ. Sci. Pollut. Res. 2018, 25, 10362–10370. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.M.; John, M.S.; Jacob, A.; Abitha, P.; Kumar, S.S.; Rajan, R.; Natarajan, S.; Pugazhendhi, A. Bactericidal coating of paper towels via sustainable biosynthesis of silver nanoparticles using Ocimum sanctum leaf extract. Mater. Res. Express 2018, 6, 045401. [Google Scholar] [CrossRef]
- Thackray, A.C.; Sammons, R.L.; Macaskie, L.E.; Yong, P.; Lugg, H.; Marquis, P.M. Bacterial biosynthesis of a calcium phosphate bone-substitute material. J. Mater. Sci. Mater. Med. 2004, 15, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Macaskie, L.E.; Yong, P.; Paterson-Beedle, M.; Thackray, A.C.; Marquis, P.M.; Sammons, R.L.; Nott, K.P.; Hall, L.D. A novel non-line-of-sight method for coating hydroxyapatite onto the surfaces of support materials by biomineralization. J. Biotechnol. 2005, 118, 187–200. [Google Scholar] [CrossRef]
- Buitrago-Vásquez, M.; Ossa-Orozco, C.P. Hydrothermal synthesis of hydroxyapatite nanorods using a fruit extract template. DYNA 2018, 85, 283–288. [Google Scholar] [CrossRef]
- Sathiyavimal, S.; Vasantharaj, S.; LewisOscar, F.; Pugazhendhi, A.; Subashkumar, R. Biosynthesis and characterization of hydroxyapatite and its composite (hydroxyapatite-gelatin-chitosan-fibrin-bone ash) for bone tissue engineering applications. Int. J. Biol. Macromol. 2019, 129, 844–852. [Google Scholar] [CrossRef]
- Wang, S.; Tian, X.; Liu, Q. The Effectiveness of Foliar Applications of Zinc and Biostimulants to Increase Zinc Concentration and Bioavailability of Wheat Grain. Agronomy 2020, 10, 178. [Google Scholar] [CrossRef] [Green Version]
- Davarpanah, S.; Tehranifar, A.; Abadia, J.; Val, J.; Davarynejad, G.; Aran, M.; Khorassani, R. Foliar calcium fertilization reduces fruit cracking in pomegranate (Punica granatum cv. Ardestani). Sci. Hortic. 2018, 230, 86–89. [Google Scholar] [CrossRef]
- Gumienna, M.; Szwengiel, A.; Górna, B. Bioactive components of pomegranate fruit and their transformation by fermentation processes. Eur. Food Res. Technol. 2016, 242, 631–640. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, P.; Mellisho, C.; Conejero, W.; Cruz, Z.; Ortuño, M.F.; Galindo, A.; Torrecillas, A. Plant water relations of leaves of pomegranate trees under different irrigation conditions. Environ. Exp. Bot. 2012, 77, 19–24. [Google Scholar] [CrossRef]
- Obireddy, S.R.; Lai, W.F. ROS-Generating Amine-Functionalized Magnetic Nanoparticles Coupled with Carboxymethyl Chitosan for pH-Responsive Release of Doxorubicin. Int. J. Nanomed. 2022, 17, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Obireddy, S.R.; Lai, W.F. Preparation and characterization of 2-hydroxyethyl starch microparticles for co-delivery of multiple bioactive agents. Drug Deliv. 2021, 28, 1562–1568. [Google Scholar] [CrossRef] [PubMed]
- Hassanisaadi, M.; Barani, M.; Rahdar, A.; Heidary, M.; Thysiadou, A.; Kyzas, G.Z. Role of agrochemical-based nanomaterials in plants: Biotic and abiotic stress with germination improvement of seeds. Plant Growth Regul. 2022, 29, 1–44. [Google Scholar] [CrossRef]
- Li, T.; Sun, M.; Wu, S. State-of-the-Art Review of Electrospun Gelatin-Based Nanofiber Dressings for Wound Healing Applications. Nanomaterials 2022, 12, 784. [Google Scholar] [CrossRef]
- Li, T.; Yin, W.; Gao, S.; Sun, Y.; Xu, P.; Wu, S.; Kong, H.; Yang, G.; Wei, G. The Combination of Two-Dimensional Nanomaterials with Metal Oxide Nanoparticles for Gas Sensors: A Review. Nanomaterials 2022, 12, 982. [Google Scholar] [CrossRef]
- Abdelmigid, H.M.; Morsi, M.M.; Hussien, N.A.; Alyamani, A.A.; Al Sufyani, N.M. Comparative Analysis of nanosilver Particles synthesized by different approaches and their antimicrobial efficacy. J. Nanomater. 2021, 2021, 12. [Google Scholar] [CrossRef]
- Tarafdar, J.C.; Raliya, R.; Rathore, I. Microbial synthesis of phosphorous nanoparticle from tri-calcium phosphate using Aspergillus tubingensis Tfr-5. J. Bionanosci. 2012, 6, 84–89. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 16, 55–63. [Google Scholar] [CrossRef]
- Metzner, H.; Rau, H.; Senger, H. Untersuchungen zur Synchronisierbareit einzelner Pigment mangel Mutanten von Chlorella. Planta 1965, 65, 186–194. [Google Scholar] [CrossRef]
- Hedge, J.E.; Hofreiter, B.T. Carbohydrate Chemistry, 17; Whistler, R.L., Be Miller, J.N., Eds.; Academic Press: New York, NY, USA, 1962; p. 420. [Google Scholar]
- Premchandra, G.S.; Saneoka, H.; Ogata, S. Cell membrane stability, an indicator of drought tolerance as affected by applied nitrogen in soybean. J. Agric. Sci. Camb. 1990, 115, 63–66. [Google Scholar] [CrossRef]
- Sairam, R.K. Effect of moisture stress on physiological activities of two contrasting wheat genotypes. Ind. J. Exp. Biol. 1994, 32, 594–597. [Google Scholar]
- Dhanda, S.S.; Sethi, G.S.; Behl, R.K. Indices of drought tolerance in wheat genotypes at early stages of plant growth. J. Agron. Crop Sci. 2004, 190, 6–12. [Google Scholar] [CrossRef]
- Juchimiuk, J.; Gnys, A.; Małuszyńska, J. DNA damage induced by mutagens in plant and human cell nuclei in the acellular comet assay. Folia Histochem. Cytobiol. 2006, 44, 127–131. [Google Scholar]
- Kalaiselvi, V.; Mathammal, R.; Vijayakumar, S.; Vaseeharan, B. Microwave-assisted green synthesis of Hydroxyapatite nanorods using Moringa oleifera flower extract and its antimicrobial applications. Int. J. Vet. Sci. Med. 2018, 6, 286–295. [Google Scholar] [CrossRef] [Green Version]
- Jalal, H.; Pal, M.A.; Ahmad, S.R.; Rather, M.; Andrabi, M.; Hamdani, S. Physico-chemical and functional properties of pomegranate peel and seed powder. Pharma Innov. J. 2018, 7, 1127–1131. [Google Scholar]
- Afriliana, A.; Hidayat, E.; Mitoma, Y.; Masuda, T.; Harada, H. Studies on Composting Spent Coffee Grounds by Aspergillus sp. and Aspergillus sp. in Aerobic Static Batch Temperature Control. J. Agric. Chem. Environ. 2021, 10, 91–112. [Google Scholar] [CrossRef]
- Bakan, F. A Systematic Study of the Effect of pH on the Initialization of Ca-deficient Hydroxyapatite to β-TCP Nanoparticles. Materials 2019, 12, 354. [Google Scholar] [CrossRef] [Green Version]
- KuKumar, G.S.; Rajendran, S.; Karthi, S.; Govindan, R.; Girija, E.K.; Karunakaran, G.; Kuznetsov, D. Green synthesis and antibacterial activity of hydroxyapatite nanorods for orthopedic applications. MRS Commun. 2017, 7, 183–188. [Google Scholar] [CrossRef] [Green Version]
- Varadarajan, V.; Varsha, M.; Vijayasekaran, K.; Shankar, S.V. Comparative studies of hydroxyapatite (HAp) nanoparticles synthesized by using different green templates. AIP Conf. Proc. 2020, 2240, 080002. [Google Scholar] [CrossRef]
- Raks, V.; Al-Suod, H.; Buszewski, B. Isolation, Separation, and Preconcentration of Biologically Active Compounds from Plant Matrices by Extraction Techniques. Chromatographia 2018, 81, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, Q.; Sun, D.; Lu, Y.; Su, Y.; Yang, X.; Wang, H.; Wang, Y.; Shao, W.; He, N.; et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology 2007, 18, 105104. [Google Scholar] [CrossRef]
- Hu, X.; Tanaka, A.; Tanaka, R. Simple extraction methods that prevent the artifactual conversion of chlorophyll to chlorophyllide during pigment isolation from leaf samples. Plant Methods 2013, 9, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Genter, R.B. Ecotoxicology of inorganic chemical stress to algae. In Algal Ecology Freshwater Benthic Ecosystems, 1st ed.; Stevenson, R.J., Bothwell, M.L., Lowe, R.L., Thorp, J., Eds.; Academic Press: San Diego, CA, USA, 1996; pp. 403–468. [Google Scholar]
- Marchiol, L.; Filippi, A.; Adamiano, A.; Degli Esposti, L.; Iafisco, M.; Mattiello, A.; Petrussa, E.; Braidot, E. Influence of Hydroxyapatite Nanoparticles on Germination and Plant Metabolism of Tomato (Solanum lycopersicum L.): Preliminary Evidence. Agronomy 2019, 9, 161. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.; Liu, X.; Li, X.; Zhao, Y. Evaluation of growth and biochemical indicators of Salvinia natans exposed to zinc oxide nanoparticles and zinc accumulation in plants. Environ. Sci. Pollut. Res. 2014, 21, 732–739. [Google Scholar] [CrossRef]
- Parida, A.; Das, B. Salt tolerance and salinity effect on plants; a review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.H.; Wang, P.F.; Hou, J.; Zhang, W.J.; Li, W.; Lin, Z.P. The effect of excess Zn on mineral nutrition and antioxidative response in rapeseed seedlings. Chemosphere 2009, 75, 1468–1476. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677–702. [Google Scholar] [CrossRef]
- Zoufan, P.; Baroonian, M.; Zargar, B. ZnO nanoparticles-induced oxidative stress in Chenopodium murale L., Zn uptake, and accumulation under hydroponic culture. Environ. Sci. Pollut. Res. 2020, 27, 11066–11078. [Google Scholar] [CrossRef]
- Salehi, H.; De Diego, N.; Chehregani Rad, A.; Benjamin, J.J.; Trevisan, M.; Lucini, L. Exogenous application of ZnO nanoparticles and ZnSO4 distinctly influence the metabolic response in Phaseolus vulgaris L. Sci. Total Environ. 2021, 778, 146331. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Kim, C. ROS-driven oxidative modification: Its impact on chloroplasts-nucleus communication. Front. Plant Sci. 2020, 10, 1729. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Mostofa, M.G.; Fujita, M. Cross protection by cold-shock to salinity and drought stress-induced oxidative stress in mustard (Brassica campestris L.) seedlings. J. Plant Sci. Mol. Breed. 2013, 4, 2. [Google Scholar] [CrossRef]
- Oloumi, H.; Soltaninejad, R.; Baghizadeh, A. The comparative effects of nano and bulk size particles of CuO and ZnO on Glycyrrhiza glabra L. seedlings. Ind. J. Plant Physiol. 2015, 20, 157–161. [Google Scholar] [CrossRef]
- Gokak, I.B.; Taranath, T.C. Morphological and biochemical responses of Abelmoschus esculantus (L.) Moench to zinc nanoparticles. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 025017. [Google Scholar] [CrossRef] [Green Version]
- Anderson, C.M.; Kohoronm, B.D. Inactivation of Arabidopsis SIPI leads to reduced levels of sugars and drought tolerance. J. Plant Physiol. 2001, 158, 1215–1219. [Google Scholar] [CrossRef]
- Sun, L.L.; Wang, Y.B.; Wang, R.L.; Wang, R.T.; Zhang, P.; Ju, Q.; Xu, J. Physiological, transcriptomic, and metabolomic analyses reveal zinc oxide nanoparticles modulate plant growth in tomato. Environ. Sci. Nano 2020, 7, 3587–3604. [Google Scholar] [CrossRef]
- Wan, J.P.; Wang, R.L.; Bai, H.R.; Wang, Y.B.; Xu, J. Comparative physiological and metabolomics analysis reveals that single-walled carbon nanohorns and ZnO nanoparticles affect salt tolerance in Sophora alopecuroides. Environ. Sci. Nano 2020, 7, 2968–2981. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, H.; White, J.; Chen, X.; Li, H.; Qu, X.; Ji, R. Metabolomics reveal that engineered nanomaterial exposure in soil alters both soil rhizosphere metabolite profiles and maize metabolic pathways. Environ. Sci. Nano 2019, 6, 1716–1727. [Google Scholar] [CrossRef]
- Wu, S.; Tian, L. Diverse Phytochemicals and Bioactivities in the Ancient Fruit and Modern Functional Food Pomegranate (Punica granatum). Molecules 2017, 22, 1606. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Liu, J.; Wang, Y.; Gao, Y.; Zhang, Z.; Xu, J.; Xing, G. Comparative physiological and metabolomic analyses revealed that foliar spraying with zinc oxide and silica nanoparticles modulates metabolite profiles in cucumber (Cucumis sativus L.). Food Energy Secur. 2021, 10, e269. [Google Scholar] [CrossRef]
- Elsayed, A.A.; Ahmed, E.G.; Taha, Z.K.; Farag, H.M.; Hussein, M.S.; AbouAitah, K. Hydroxyapatite nanoparticles as novel nano-fertilizer for production of rosemary plants. Sci. Hortic. 2022, 295, 110851. [Google Scholar] [CrossRef]
- Ramis, J.M.; Coelho, C.C.; Córdoba, A.; Quadros, P.A.; Monjo, M. Safety Assessment of Nano-Hydroxyapatite as an Oral Care Ingredient according to the EU Cosmetics Regulation. Cosmetics 2018, 5, 53. [Google Scholar] [CrossRef] [Green Version]
- Remya, N.S.; Syama, S.; Gayathri, V.; Varma, H.K.; Mohanan, P.V. An in vitro study on the interaction of hydroxyapatite nanoparticles and bone marrow mesenchymal stem cells for assessing the toxicological behaviour. Colloids Surf. B Biointerfaces 2014, 117, 389–397. [Google Scholar] [CrossRef]
- Sonmez, E.; Cacciatore, I.; Bakan, F.; Turkez, H.; Mohtar, Y.I.; Togar, B.; Stefano, A.D. Toxicity assessment of hydroxyapatite nanoparticles in rat liver cell model in vitro. Hum. Exp. Toxicol. 2016, 35, 1073–1083. [Google Scholar] [CrossRef]
- Saed-Moucheshi, A.; Hasheminasab, H.; Khaledian, Z.; Pessarakli, M. Exploring morpho-physiological relationships among drought resistance related traits in wheat genotypes using multivariate techniques. J. Plant Nutr. 2015, 38, 2077–2095. [Google Scholar] [CrossRef]
- Maghsoodi, M.R.; Najafi, N.; Reyhanitabar, A.; Oustan, S. Hydroxyapatite nanorods, hydrochar, biochar, and zeolite for controlled-release urea fertilizers. Geoderma 2020, 379, 114644. [Google Scholar] [CrossRef]
- Gonzalez, L.; Lison, D.; Kirsch-Volders, M. Genotoxicity of engineered nanomaterials: A critical review. Nanotoxicology 2008, 2, 252–273. [Google Scholar] [CrossRef]
- AshaRrani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Ghosh, M.; Bandyopadhyay, M.; Mukherjee, A. Genotoxicity of titanium dioxide (TiO2) nanoparticles at two trophic levels: Plant and human lymphocytes. Chemosphere 2010, 81, 1253–1262. [Google Scholar] [CrossRef]
- Kumari, M.; Mukherjee, A.; Chandrasekaran, N. Genotoxicity of silver nanoparticles in Allium cepa. Sci. Total Environ. 2009, 407, 5243–5246. [Google Scholar] [CrossRef] [PubMed]
- Wise, J.P., Sr.; Goodale, B.C.; Wise, S.S.; Craig, G.A.; Pongan, A.F.; Walter, R.B.; Thompson, W.D.; Ng, A.-K.; Aboueissa, A.E.-M.; Mitani, H.; et al. Silver nanospheres are cytotoxic and genotoxic to fish cells. Aquat. Toxicol. 2010, 97, 34–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, A.R. The comet assay for DNA damage and repair: Principles, applications, and limitations. Mol. Biotechnol. 2004, 26, 249–261. [Google Scholar] [CrossRef]
- Ferraro, D.; Anselmi-Tamburini, U.; Tredici, I.G.; Ricci, V.; Sommi, P. Overestimation of nanoparticles-induced DNA damage determined by the comet assay. Nanotoxicology 2016, 10, 861–870. [Google Scholar] [CrossRef]
- Glei, M.; Schneider, T.; Schlormann, W. Comet assay: An essential tool in toxicological research. Arch. Toxicol. 2016, 90, 2315–2336. [Google Scholar] [CrossRef]
- Vandghanooni, S.; Eskandani, M. Comet assay: A method to evaluate genotoxicity of nano-drug delivery system. Bioimpacts 2011, 1, 87–97. [Google Scholar]
- Tice, R.R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.C.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagenes. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Saquib, Q.; Faisal, M.; Alatar, A.A.; Al-Khedhairy, A.A.; Ahmed, M.; Ansari, S.M.; Alwathnani, H.A.; Okla, M.K.; Dwivedi, S.; Musarrat, J.; et al. Genotoxicity of ferric oxide nanoparticles in Raphanus sativus: Deciphering the role of signaling factors, oxidative stress and cell death. J. Environ. Sci. 2013, 47, 49–62. [Google Scholar] [CrossRef]
- Ghosh, M.; Ghosh, I.; Godderis, L.; Hoet, P.; Mukherjee, A. Genotoxicity of engineered nanoparticles in higher plants. Mutat. Res. Genet. Toxicol. Environ. Mutagenes. 2019, 842, 132–145. [Google Scholar] [CrossRef]
- Petrova, A.; Plaksenkova, I.; Kokina, I.; Jermaļonoka, M. Effect of Fe3O4 and CuO Nanoparticles on Morphology, Genotoxicity, and miRNA Expression on Different Barley (Hordeum vulgare L.) Genotypes. Sci. World J. 2021, 2021, 6644689. [Google Scholar] [CrossRef]
- Konate, A.; He, X.; Zhang, Z.; Ma, Y.; Zhang, P.; Alugongo, G.M.; Rui, Y. Magnetic (Fe3O4) nanoparticles reduce heavy metals uptake and mitigate their toxicity in wheat seedling. Sustainability 2017, 9, 790. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Ma, C.; White, J.C.; Xing, B. Effects of Phosphorus Ensembled Nanomaterials on Nutrient Uptake and Distribution in Glycine max L. under Simulated Precipitation. Agronomy 2021, 11, 1086. [Google Scholar] [CrossRef]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelmigid, H.M.; Morsi, M.M.; Hussien, N.A.; Alyamani, A.A.; Alhuthal, N.A.; Albukhaty, S. Green Synthesis of Phosphorous-Containing Hydroxyapatite Nanoparticles (nHAP) as a Novel Nano-Fertilizer: Preliminary Assessment on Pomegranate (Punica granatum L.). Nanomaterials 2022, 12, 1527. https://doi.org/10.3390/nano12091527
Abdelmigid HM, Morsi MM, Hussien NA, Alyamani AA, Alhuthal NA, Albukhaty S. Green Synthesis of Phosphorous-Containing Hydroxyapatite Nanoparticles (nHAP) as a Novel Nano-Fertilizer: Preliminary Assessment on Pomegranate (Punica granatum L.). Nanomaterials. 2022; 12(9):1527. https://doi.org/10.3390/nano12091527
Chicago/Turabian StyleAbdelmigid, Hala M., Maissa M. Morsi, Nahed Ahmed Hussien, Amal Ahmed Alyamani, Nawal Abdallah Alhuthal, and Salim Albukhaty. 2022. "Green Synthesis of Phosphorous-Containing Hydroxyapatite Nanoparticles (nHAP) as a Novel Nano-Fertilizer: Preliminary Assessment on Pomegranate (Punica granatum L.)" Nanomaterials 12, no. 9: 1527. https://doi.org/10.3390/nano12091527
APA StyleAbdelmigid, H. M., Morsi, M. M., Hussien, N. A., Alyamani, A. A., Alhuthal, N. A., & Albukhaty, S. (2022). Green Synthesis of Phosphorous-Containing Hydroxyapatite Nanoparticles (nHAP) as a Novel Nano-Fertilizer: Preliminary Assessment on Pomegranate (Punica granatum L.). Nanomaterials, 12(9), 1527. https://doi.org/10.3390/nano12091527