Transport and Retention of Poly(Acrylic Acid-co-Maleic Acid) Coated Magnetite Nanoparticles in Porous Media: Effect of Input Concentration, Ionic Strength and Grain Size
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fixed–Bed Column Setup
2.3. PAM@MNP Column Experiments
2.4. Mathematical Modeling
3. Results and Discussion
3.1. Characteristics of PAM@MNP
3.2. Effect of Initial Concentration on PAM@MNP Transport and Retention
3.3. Effect of Ionic Strength and Initial Concentration on PAM@MNP Transport and Retention
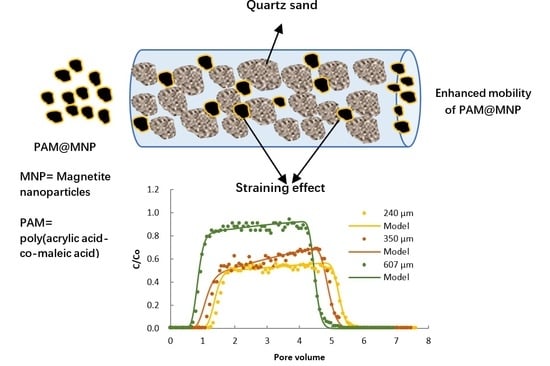
3.4. Effect of Grain Size on the Transport and Retention of PAM@MNP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vallabani, N.V.S.; Singh, S. Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics. 3 Biotech 2018, 8, 279. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Wang, J.; Lin, Y.; Wang, J. Nanoparticle-based biosensors and bioassays. In Electrochemical Sensors, Biosensors and their Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Pasinszki, T.; Krebsz, M. Synthesis and Application of Zero-Valent Iron Nanoparticles in Water Treatment, Environmental Remediation, Catalysis, and Their Biological Effects. Nanomaterials 2020, 10, 917. [Google Scholar] [CrossRef] [PubMed]
- Galdames, A.; Ruiz-Rubio, L.; Orueta, M.; Sánchez-Arzalluz, M.; Vilas-Vilela, J.L. Zero-Valent Iron Nanoparticles for Soil and Groundwater Remediation. Int. J. Environ. Res. Public Health 2020, 17, 5817. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K. Application of Nanotechnology in Water Research; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Yan, W.; Lien, H.-L.; Koel, B.E.; Zhang, W.-X. Iron nanoparticles for environmental clean-up: Recent developments and future outlook. Environ. Sci. Process. Impacts 2013, 15, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Cecchin, I.; Reddy, K.; Thomé, A.; Tessaro, E.F.; Schnaid, F. Nanobioremediation: Integration of nanoparticles and bioremediation for sustainable remediation of chlorinated organic contaminants in soils. Int. Biodeterior. Biodegrad. 2017, 119, 419–428. [Google Scholar] [CrossRef]
- Li, Q.; Chen, X.; Zhuang, J.; Chen, X. Decontaminating soil organic pollutants with manufactured nanoparticles. Environ. Sci. Pollut. Res. 2016, 23, 11533–11548. [Google Scholar] [CrossRef]
- Pillai, H.P.S.; Kottekottil, J. Nano-Phytotechnological Remediation of Endosulfan Using Zero Valent Iron Nanoparticles. J. Environ. Prot. 2016, 7, 734–744. [Google Scholar] [CrossRef] [Green Version]
- Rani, M.; Shanker, U.; Jassal, V. Recent strategies for removal and degradation of persistent & toxic organochlorine pesticides using nanoparticles: A review. J. Environ. Manag. 2017, 190, 208–222. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Siddiqui, M.T.H.; Mubarak, N.M.; Baloch, H.A.; Abdullah, E.C.; Mazari, S.A.; Griffin, G.J.; Srinivasan, M.P.; Tanksale, A. Chapter 17—Iron Oxide Nanomaterials for the Removal of Heavy Metals and Dyes From Wastewater. In Micro and Nano Technologies; Thomas, S., Pasquini, D., Leu, S.-Y., Gopakumar, D.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 447–472. [Google Scholar]
- Bhateria, R.; Singh, R. A review on nanotechnological application of magnetic iron oxides for heavy metal removal. J. Water Process Eng. 2019, 31, 100845. [Google Scholar] [CrossRef]
- Cheng, Z.; Tan, A.L.K.; Tao, Y.; Shan, D.; Ting, K.E.; Yin, X.J. Synthesis and Characterization of Iron Oxide Nanoparticles and Applications in the Removal of Heavy Metals from Industrial Wastewater. Int. J. Photoenergy 2012, 2012, 608298. [Google Scholar] [CrossRef]
- Samrot, A.V.; Sahithya, C.S.; Selvarani, J.; Pachiyappan, S. Surface-Engineered Super-Paramagnetic Iron Oxide Nanoparticles For Chromium Removal. Int. J. Nanomed. 2019, 14, 8105–8119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baragaño, D.; Alonso, J.; Gallego, J.; Lobo, M.; Gil-Díaz, M. Magnetite nanoparticles for the remediation of soils co-contaminated with As and PAHs. Chem. Eng. J. 2020, 399, 125809. [Google Scholar] [CrossRef]
- Giraldo, L.; Erto, A.; Moreno-Piraján, J.C. Magnetite nanoparticles for removal of heavy metals from aqueous solutions: Synthesis and characterization. Adsorption 2013, 19, 465–474. [Google Scholar] [CrossRef]
- Huang, Y.; Keller, A.A. Magnetic Nanoparticle Adsorbents for Emerging Organic Contaminants. ACS Sustain. Chem. Eng. 2013, 1, 731–736. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Kong, J. Novel magnetic Fe3O4@C nanoparticles as adsorbents for removal of organic dyes from aqueous solution. J. Hazard. Mater. 2011, 193, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Aggarwal, I.; Kumar, H.; Prasad, L.; Kumar, A.; Sharma, A.; Vo, D.-V.N.; Van Thuan, D.; Mishra, V. Magnetite nanoparticles as sorbents for dye removal: A review. Environ. Chem. Lett. 2021, 19, 2487–2525. [Google Scholar] [CrossRef]
- Su, C. Environmental implications and applications of engineered nanoscale magnetite and its hybrid nanocomposites: A review of recent literature. J. Hazard. Mater. 2017, 322, 48–84. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Chircov, C.; Grumezescu, A.M. Magnetite nanoparticles: Synthesis methods—A comparative review. Methods 2021, 199, 16–27. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Roy, E.; Patra, S.; Karfa, P.; Madhuri, R.; Sharma, P.K. Role of Magnetic Nanoparticles in Providing Safe and Clean Water to Each Individual BT—Complex Magnetic Nanostructures: Synthesis, Assembly and Applications; Sharma, S.K., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 281–316. [Google Scholar]
- Tóth, I.Y.; Illés, E.; Bauer, R.A.; Nesztor, D.; Szekeres, M.; Zupkó, I.; Tombácz, E. Designed Polyelectrolyte Shell on Magnetite Nanocore for Dilution-Resistant Biocompatible Magnetic Fluids. Langmuir 2012, 28, 16638–16646. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; He, Q.; Jiang, C. Magnetic Iron Oxide Nanoparticles: Synthesis and Surface Functionalization Strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krenkova, J.; Foret, F. Iron oxide nanoparticle coating of organic polymer-based monolithic columns for phosphopeptide enrichment. J. Sep. Sci. 2011, 34, 2106–2112. [Google Scholar] [CrossRef] [PubMed]
- Phenrat, T.; Cihan, A.; Kim, H.-J.; Mital, M.; Illangasekare, T.; Lowry, G.V. Transport and Deposition of Polymer-Modified Fe0 Nanoparticles in 2-D Heterogeneous Porous Media: Effects of Particle Concentration, Fe0 Content, and Coatings. Environ. Sci. Technol. 2010, 44, 9086–9093. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-D.; Zevi, Y.; Kou, X.-M.; Xiao, J.; Wang, X.-J.; Jin, Y. Effect of dissolved organic matter on the stability of magnetite nanoparticles under different pH and ionic strength conditions. Sci. Total Environ. 2010, 408, 3477–3489. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Mikhaylova, M.; Zhang, Y.; Muhammed, M. Protective Coating of Superparamagnetic Iron Oxide Nanoparticles. Chem. Mater. 2003, 15, 1617–1627. [Google Scholar] [CrossRef]
- Elimelech, M.; O’Melia, C.R. Kinetics of deposition of colloidal particles in porous media. Environ. Sci. Technol. 1990, 24, 1528–1536. [Google Scholar] [CrossRef]
- Pham, N.H.; Papavassiliou, D.V. Nanoparticle transport in heterogeneous porous media with particle tracking numerical methods. Comput. Part. Mech. 2017, 4, 87–100. [Google Scholar] [CrossRef]
- Bradford, S.A.; Simunek, J.; Bettahar, M.; van Genuchten, M.; Yates, S.R. Significance of straining in colloid deposition: Evidence and implications. Water Resour. Res. 2006, 42, 1–16. [Google Scholar] [CrossRef]
- Raychoudhury, T.; Tufenkji, N.; Ghoshal, S. Straining of polyelectrolyte-stabilized nanoscale zero valent iron particles during transport through granular porous media. Water Res. 2014, 50, 80–89. [Google Scholar] [CrossRef] [Green Version]
- Tufenkji, N.; Elimelech, M. Correlation Equation for Predicting Single-Collector Efficiency in Physicochemical Filtration in Saturated Porous Media. Environ. Sci. Technol. 2004, 38, 529–536. [Google Scholar] [CrossRef]
- Bradford, S.A.; Bettahar, M.; Simunek, J.; van Genuchten, M. Straining and Attachment of Colloids in Physically Heterogeneous Porous Media. Vadose Zone J. 2004, 3, 384–394. [Google Scholar] [CrossRef]
- Liang, Y.; Zhou, J.; Dong, Y.; Klumpp, E.; Šimůnek, J.; Bradford, S.A. Evidence for the critical role of nanoscale surface roughness on the retention and release of silver nanoparticles in porous media. Environ. Pollut. 2020, 258, 113803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Bradford, S.A.; Šimůnek, J.; Vereecken, H.; Klumpp, E. Co-transport of multi-walled carbon nanotubes and sodium dodecylbenzenesulfonate in chemically heterogeneous porous media. Environ. Pollut. 2019, 247, 907–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Gao, B.; Tang, D. Review of key factors controlling engineered nanoparticle transport in porous media. J. Hazard. Mater. 2016, 318, 233–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, C.; Lazouskaya, V.; Zhang, H.; Li, B.; Jin, Y.; Huang, Y. Influence of surface chemical heterogeneity on attachment and detachment of microparticles. Colloids Surf. A Physicochem. Eng. Asp. 2013, 433, 14–29. [Google Scholar] [CrossRef]
- Lin, D.; Tian, X.; Wu, F.; Xing, B. Fate and Transport of Engineered Nanomaterials in the Environment. J. Environ. Qual. 2010, 39, 1896–1908. [Google Scholar] [CrossRef] [Green Version]
- Kuhnena, F.; Barmettlera, K.; Bhattacharjeeb, S.; Elimelech, M.; Kretzschmar, R. Transport of Iron Oxide Colloids in Packed Quartz Sand Media: Monolayer and Multilayer Deposition. J. Colloid Interface Sci. 2000, 231, 32–41. [Google Scholar] [CrossRef]
- Shipley, H.J.; Yean, S.; Kan, A.T.; Tomson, M.B. Adsorption of Arsenic to Magnetite Nanoparticles: Effect of Particle Concentration, Ph, Ionic Strength, and Temperature. Environ. Toxicol. Chem. 2009, 28, 509–515. [Google Scholar] [CrossRef]
- Tosco, T.A.E.; Bosch, J.; Meckenstock, R.U.; Sethi, R. Transport of Ferrihydrite Nanoparticles in Saturated Porous Media: Role of Ionic Strength and Flow Rate. Environ. Sci. Technol. 2012, 46, 4008–4015. [Google Scholar] [CrossRef] [Green Version]
- Baalousha, M. Aggregation and disaggregation of iron oxide nanoparticles: Influence of particle concentration, pH and natural organic matter. Sci. Total Environ. 2009, 407, 2093–2101. [Google Scholar] [CrossRef]
- Tiraferri, A.; Chen, K.L.; Sethi, R.; Elimelech, M. Reduced aggregation and sedimentation of zero-valent iron nanoparticles in the presence of guar gum. J. Colloid Interface Sci. 2008, 324, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Horst, M.F.; Lassalle, V.; Ferreira, M.L. Nanosized magnetite in low cost materials for remediation of water polluted with toxic metals, azo- and antraquinonic dyes. Front. Environ. Sci. Eng. 2015, 9, 746–769. [Google Scholar] [CrossRef]
- Soares, S.F.; Fernandes, T.; Trindade, T.; Daniel-Da-Silva, A.L. Recent advances on magnetic biosorbents and their applications for water treatment. Environ. Chem. Lett. 2020, 18, 151–164. [Google Scholar] [CrossRef]
- Pan, G.; Li, L.; Zhao, D.; Chen, H. Immobilization of non-point phosphorus using stabilized magnetite nanoparticles with enhanced transportability and reactivity in soils. Environ. Pollut. 2010, 158, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Park, C.M.; Masud, A.; Aich, N.; Su, C. Carboxymethylcellulose Mediates the Transport of Carbon Nanotube—Magnetite Nanohybrid Aggregates in Water-Saturated Porous Media. Environ. Sci. Technol. 2017, 51, 12405–12415. [Google Scholar] [CrossRef]
- Ersenkal, D.A.; Ziylana, A.; Ince, N.H.; Acar, H.Y.; Demirerb, M.; Copty, N.K. Impact of dilution on the transport of poly(acrylic acid) supported magnetite nanoparticles in porous media. J. Contam. Hydrol. 2011, 126, 248–257. [Google Scholar] [CrossRef]
- Golzar, M.; Saghravani, S.F.; Moghaddam, M.A. Experimental Study and Numerical Solution of Poly Acrylic Acid Supported Magnetite Nanoparticles Transport in a One-Dimensional Porous Media. Adv. Mater. Sci. Eng. 2014, 2014, 864068. [Google Scholar] [CrossRef] [Green Version]
- Kmetz, A.A.; Becker, M.D.; Lyon, B.A.; Foster, E.; Xue, Z.; Johnston, K.P.; Abriola, L.M.; Pennell, K.D. Improved Mobility of Magnetite Nanoparticles at High Salinity with Polymers and Surfactants. Energy Fuels 2016, 30, 1915–1926. [Google Scholar] [CrossRef]
- Ureña-Benavides, E.E.; Lin, E.L.; Foster, E.L.; Xue, Z.; Ortiz, M.R.; Fei, Y.; Larsen, E.S.; Ii, A.A.K.; Lyon, B.A.; Moaseri, E.; et al. Low Adsorption of Magnetite Nanoparticles with Uniform Polyelectrolyte Coatings in Concentrated Brine on Model Silica and Sandstone. Ind. Eng. Chem. Res. 2016, 55, 1522–1532. [Google Scholar] [CrossRef]
- Xue, Z.; Foster, E.; Wang, Y.; Nayak, S.; Cheng, V.; Ngo, V.W.; Pennell, K.D.; Bielawski, C.W.; Johnston, K.P. Effect of Grafted Copolymer Composition on Iron Oxide Nanoparticle Stability and Transport in Porous Media at High Salinity. Energy Fuels 2014, 28, 3655–3665. [Google Scholar] [CrossRef]
- Becker, M.D.; Wang, Y.; Paulsen, J.L.; Song, Y.-Q.; Abriola, L.M.; Pennell, K.D. In situ measurement and simulation of nano-magnetite mobility in porous media subject to transient salinity. Nanoscale 2015, 7, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Lyon-Marion, B.A.; Becker, M.D.; Kmetz, A.A.; Foster, E.; Johnston, K.P.; Abriola, L.M.; Pennell, K.D. Simulation of magnetite nanoparticle mobility in a heterogeneous flow cell. Environ. Sci. Nano 2017, 4, 1512–1524. [Google Scholar] [CrossRef]
- Felthouse, T.R.; Burnett, J.C.; Horrell, B.; Mummey, M.J.; Kuo, Y.-J. Maleic Anhydride, Maleic Acid, and Fumaric Acid. Kirk-Othmer Encycl. Chem. Technol. 2001. [Google Scholar] [CrossRef]
- Hsiou, Y.; Wang, Y.; Liu, L.-K. Structures of tetracarbonyl (2–3-η-maleic acid) iron, cis-[Fe (C4H4O4)(CO)4](1) and tetracarbonyl (2–3-η-fumaric acid) iron, trans-[Fe (C4H4O4)(CO)4](2). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1989, 45, 721–724. [Google Scholar] [CrossRef]
- Bychkova, S.A.; Katrovtseva, A.V.; Kozlovskii, E.V. The potentiometric study of maleic acid complexation with the alkaline-earth metal ions in aqueous solutions. Russ. J. Coord. Chem. 2008, 34, 172–174. [Google Scholar] [CrossRef]
- Tombácz, E.; Tóth, I.Y.; Nesztor, D.; Illés, E.; Hajdú, A.; Szekeres, M.; Vékás, L. Adsorption of organic acids on magnetite nanoparticles, pH-dependent colloidal stability and salt tolerance. Colloids Surf. A Physicochem. Eng. Asp. 2013, 435, 91–96. [Google Scholar] [CrossRef] [Green Version]
- Kasel, D.; Bradford, S.A.; Šimůnek, J.; Heggen, M.; Vereecken, H.; Klumpp, E. Transport and retention of multi-walled carbon nanotubes in saturated porous media: Effects of input concentration and grain size. Water Res. 2013, 47, 933–944. [Google Scholar] [CrossRef]
- Illés, E.; Tombácz, E. The effect of humic acid adsorption on pH-dependent surface charging and aggregation of magnetite nanoparticles. J. Colloid Interface Sci. 2006, 295, 115–123. [Google Scholar] [CrossRef]
- Šimůnek, J.; van Genuchten, M.T.; Šejna, M. Development and Applications of the HYDRUS and STANMOD Software Packages and Related Codes. Vadose Zone J. 2008, 7, 587–600. [Google Scholar] [CrossRef] [Green Version]
- Gargiulo, G.; Bradford, S.; Šimůnek, J.; Ustohal, P.; Vereecken, H.; Klumpp, E. Bacteria transport and deposition under unsaturated conditions: The role of the matrix grain size and the bacteria surface protein. J. Contam. Hydrol. 2007, 92, 255–273. [Google Scholar] [CrossRef]
- Deshpande, P.A.; Shonnard, D.R. Modeling the effects of systematic variation in ionic strength on the attachment kinetics ofPseudomonas fluorescensUPER-1 in saturated sand columns. Water Resour. Res. 1999, 35, 1619–1627. [Google Scholar] [CrossRef]
- Bradford, S.A.; Simunek, J.; Bettahar, M.; van Genuchten, M.T.; Yates, S.R. Modeling Colloid Attachment, Straining, and Exclusion in Saturated Porous Media. Environ. Sci. Technol. 2003, 37, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, D.W. An Algorithm for Least-Squares Estimation of Nonlinear Parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Zhang, M.; He, F.; Zhao, D.; Hao, X. Transport of stabilized iron nanoparticles in porous media: Effects of surface and solution chemistry and role of adsorption. J. Hazard. Mater. 2017, 322, 284–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degenkolb, L.; Dippon, U.; Pabst, S.; Klitzke, S. Transport and retention of differently coated CeO2 nanoparticles in saturated sediment columns under laboratory and near-natural conditions. Environ. Sci. Pollut. Res. 2019, 26, 15905–15919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Ge, L.; He, J.; Zhang, W.; Jaisi, D.P.; Zhou, D. Hyperexponential and nonmonotonic retention of polyvinylpyrrolidone-coated silver nanoparticles in an Ultisol. J. Contam. Hydrol. 2014, 164, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Bradford, S.A.; Simunek, J.; Vereecken, H.; Klumpp, E. Sensitivity of the transport and retention of stabilized silver nanoparticles to physicochemical factors. Water Res. 2013, 47, 2572–2582. [Google Scholar] [CrossRef] [PubMed]
- Rahmatpour, S.; Mosaddeghi, M.R.; Shirvani, M.; Šimůnek, J. Transport of silver nanoparticles in intact columns of calcareous soils: The role of flow conditions and soil texture. Geoderma 2018, 322, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.; Honda, R.J.; Myung, N.V.; Walker, S.L. Transport of Iron-Based Nanoparticles: Role of Magnetic Properties. Environ. Sci. Technol. 2009, 43, 8834–8839. [Google Scholar] [CrossRef]
- Rahman, T.; Millwater, H.; Shipley, H.J. Modeling and sensitivity analysis on the transport of aluminum oxide nanoparticles in saturated sand: Effects of ionic strength, flow rate, and nanoparticle concentration. Sci. Total Environ. 2014, 499, 402–412. [Google Scholar] [CrossRef]
- Wang, C.; Bobba, A.D.; Attinti, R.; Shen, C.; Lazouskaya, V.; Wang, L.-P.; Jin, Y. Retention and Transport of Silica Nanoparticles in Saturated Porous Media: Effect of Concentration and Particle Size. Environ. Sci. Technol. 2012, 46, 7151–7158. [Google Scholar] [CrossRef]
- Saleh, N.; Kim, H.-J.; Phenrat, T.; Matyjaszewski, K.; Tilton, R.D.; Lowry, G.V. Ionic Strength and Composition Affect the Mobility of Surface-Modified Fe0 Nanoparticles in Water-Saturated Sand Columns. Environ. Sci. Technol. 2008, 42, 3349–3355. [Google Scholar] [CrossRef] [PubMed]
- French, R.A.; Jacobson, A.R.; Kim, B.; Isley, S.L.; Penn, R.L.; Baveye, P. Influence of Ionic Strength, pH, and Cation Valence on Aggregation Kinetics of Titanium Dioxide Nanoparticles. Environ. Sci. Technol. 2009, 43, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- El Badawy, A.M.; Luxton, T.P.; Silva, R.G.; Scheckel, K.; Suidan, M.T.; Tolaymat, T.M. Impact of Environmental Conditions (pH, Ionic Strength, and Electrolyte Type) on the Surface Charge and Aggregation of Silver Nanoparticles Suspensions. Environ. Sci. Technol. 2010, 44, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Napper, D. Steric stabilization. J. Colloid Interface Sci. 1977, 58, 390–407. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, B.; Bradford, S.A.; Wu, L.; Chen, H.; Shi, X.; Wu, J. Transport, retention, and size perturbation of graphene oxide in saturated porous media: Effects of input concentration and grain size. Water Res. 2015, 68, 24–33. [Google Scholar] [CrossRef]
- Lu, H.; Dong, J.; Xi, B.; Cai, P.; Xia, T.; Zhang, M. Transport and retention of porous silicon-coated zero-valent iron in saturated porous media. Environ. Pollut. 2021, 276, 116700. [Google Scholar] [CrossRef]
- Saberinasr, A.; Rezaei, M.; Nakhaei, M.; Hosseini, S.M. Transport of CMC-Stabilized nZVI in Saturated Sand Column: The Effect of Particle Concentration and Soil Grain Size. Water Air Soil Pollut. 2016, 227, 394. [Google Scholar] [CrossRef]
- Bradford, S.A.; Bettahar, M. Concentration dependent transport of colloids in saturated porous media. J. Contam. Hydrol. 2006, 82, 99–117. [Google Scholar] [CrossRef]
- Bradford, S.A.; Torkzaban, S.; Simunek, J. Modeling colloid transport and retention in saturated porous media under unfavorable attachment conditions. Water Resour. Res. 2011, 47, W10503. [Google Scholar] [CrossRef]
- Liang, Y.; Luo, Y.; Lu, Z.; Klumpp, E.; Shen, C.; Bradford, S.A. Evidence on enhanced transport and release of silver nanoparticles by colloids in soil due to modification of grain surface morphology and co-transport. Environ. Pollut. 2021, 276, 116661. [Google Scholar] [CrossRef] [PubMed]




| Figure | d50 (µm) | Co (mg L−1) | IS (mM) | q (cm min−1) | φ | λ cm | ζ Potential of NP (mV) | Zave-NP (nm) |
|---|---|---|---|---|---|---|---|---|
| 1 | 607 | 1 | 1 | 0.29 | 0.38 | 0.146 | −62.1 ± 3.4 | 126.2 ± 1.5 |
| 607 | 5 | 1 | 0.28 | 0.34 | 0.171 | −66.1 ± 2.7 | 128.7 ± 1.5 | |
| 607 | 10 | 1 | 0.29 | 0.34 | 0.175 | −73.0 ± 1.8 | 121.0 ± 0.8 | |
| 2 | 607 | 1 | 1 | 0.29 | 0.38 | 0.146 | −62.1 ± 3.4 | 126.2 ± 1.5 |
| 607 | 1 | 5 | 0.29 | 0.39 | 0.138 | −59.1 ± 4.8 | 128.2 ± 3.0 | |
| 607 | 1 | 10 | 0.30 | 0.36 | 0.137 | −27.1 ± 4.7 | 119.3 ± 0.6 | |
| 3 | 607 | 10 | 1 | 0.29 | 0.34 | 0.175 | −73.0 ± 1.8 | 121.0 ± 0.8 |
| 607 | 10 | 5 | 0.30 | 0.36 | 0.154 | −64.0 ± 1.4 | 122.1 ± 1.8 | |
| 607 | 10 | 10 | 0.28 | 0.39 | 0.153 | −43.7 ± 2.1 | 121.6 ± 1.2 | |
| 607 | 10 | 50 | 0.30 | 0.43 | 0.953 | −51.2 ± 3.6 | 115.4 ± 0.5 | |
| 607 | 10 | 100 | 0.30 | 0.38 | 1.887 | −53.2 ± 2.3 | 115.5 ± 0.2 | |
| 4 | 607 | 10 | 1 | 0.29 | 0.34 | 0.175 | −73.0 ± 1.8 | 121.0 ± 0.8 |
| 350 | 10 | 1 | 0.28 | 0.35 | 0.213 | −73.0 ± 1.8 | 121.0 ± 0.8 | |
| 240 | 10 | 1 | 0.28 | 0.35 | 0.094 | −73.0 ± 1.8 | 121.0 ± 0.8 |
| Figure | Co [mg L−1] | dc [µm] | IS [mM] | q [cm min −1] | k1 [min −1] | SE k1 | Smax/Co [cm3 g−1] | SE Smax/Co | R2 | Recovery % | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Meff | Msand | Mtotal | ||||||||||
| 1 | 1 | 607 | 1 | 0.29 | 0.25 | 0.02 | 0.58 | 0.06 | 0.94 | 65.6 | 27.7 | 93.3 |
| 5 | 607 | 1 | 0.28 | 0.08 | 0.00 | 0.27 | 0.03 | 0.99 | 84.3 | 18.2 | 95.9 | |
| 10 | 607 | 1 | 0.29 | 0.09 | 0.01 | 0.18 | 0.02 | 0.99 | 88.4 | 5.7 | 94.1 | |
| 2 | 1 | 607 | 1 | 0.29 | 0.23 | 0.01 | 0.57 | 0.06 | 0.95 | 65.6 | 27.7 | 93.3 |
| 1 | 607 | 5 | 0.29 | 0.23 | 0.01 | 1.63 | 0.27 | 0.90 | 58.1 | 26.8 | 84.8 | |
| 1 | 607 | 10 | 0.30 | 0.25 | 0.02 | 1.65 | 0.49 | 0.87 | 46.4 | 39.8 | 86.2 | |
| 3 | 10 | 607 | 1 | 0.29 | 0.09 | 0.01 | 0.18 | 0.02 | 0.99 | 88.4 | 5.7 | 94.1 |
| 10 | 607 | 5 | 0.30 | 0.06 | 0.01 | 0.71 | 0.58 | 0.98 | 85.7 | 4.8 | 90.6 | |
| 10 | 607 | 10 | 0.28 | 0.08 | 0.01 | 0.06 | 0.01 | 0.98 | 99.3 | 5.3 | 104.6 | |
| 10 | 607 | 50 | 0.30 | 0.11 | 0.01 | 0.35 | 0.07 | 0.96 | 79.4 | 5.3 | 84.7 | |
| 10 | 607 | 100 | 0.30 | 0.03 | 0.01 | 0.05 | 0.01 | 0.97 | 90.4 | 5.3 | 95.7 | |
| 4 | 10 | 240 | 1 | 0.29 | 0.34 | 0.01 | 2.87 | 0.51 | 0.90 | 45.2 | 36.9 | 82.1 |
| 10 | 350 | 1 | 0.28 | 0.38 | 0.03 | 0.48 | 0.05 | 0.94 | 56.7 | 45.5 | 102 | |
| 10 | 607 | 1 | 0.28 | 0.09 | 0.01 | 0.18 | 0.02 | 0.99 | 88.4 | 5.7 | 94.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mlih, R.; Liang, Y.; Zhang, M.; Tombácz, E.; Bol, R.; Klumpp, E. Transport and Retention of Poly(Acrylic Acid-co-Maleic Acid) Coated Magnetite Nanoparticles in Porous Media: Effect of Input Concentration, Ionic Strength and Grain Size. Nanomaterials 2022, 12, 1536. https://doi.org/10.3390/nano12091536
Mlih R, Liang Y, Zhang M, Tombácz E, Bol R, Klumpp E. Transport and Retention of Poly(Acrylic Acid-co-Maleic Acid) Coated Magnetite Nanoparticles in Porous Media: Effect of Input Concentration, Ionic Strength and Grain Size. Nanomaterials. 2022; 12(9):1536. https://doi.org/10.3390/nano12091536
Chicago/Turabian StyleMlih, Rawan, Yan Liang, Miaoyue Zhang, Etelka Tombácz, Roland Bol, and Erwin Klumpp. 2022. "Transport and Retention of Poly(Acrylic Acid-co-Maleic Acid) Coated Magnetite Nanoparticles in Porous Media: Effect of Input Concentration, Ionic Strength and Grain Size" Nanomaterials 12, no. 9: 1536. https://doi.org/10.3390/nano12091536
APA StyleMlih, R., Liang, Y., Zhang, M., Tombácz, E., Bol, R., & Klumpp, E. (2022). Transport and Retention of Poly(Acrylic Acid-co-Maleic Acid) Coated Magnetite Nanoparticles in Porous Media: Effect of Input Concentration, Ionic Strength and Grain Size. Nanomaterials, 12(9), 1536. https://doi.org/10.3390/nano12091536