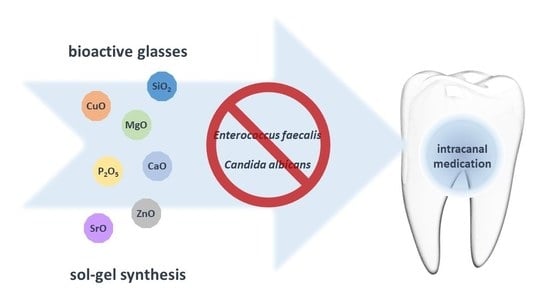
New and Efficient Bioactive Glass Compositions for Controlling Endodontic Pathogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Bioactive Glass Samples
2.2. Thermal Treatment and Milling
2.3. Physical and Morphological Characterization of the BG Samples
2.4. Evaluation of Antimicrobial Activity
2.4.1. Microbial Strains and Growth Conditions
2.4.2. Growth Inhibition Effect of BG on E. faecalis and C. albicans
2.5. Statistical Analysis
3. Results
3.1. Physical and Morphological Characterization of BG Samples
3.1.1. Thermal Behavior
3.1.2. Crystalline Phases
3.1.3. Chemical Functional Groups
3.1.4. Morphology, Surface Area, and Particle Size
3.2. Evaluation of Antimicrobial Activity
Growth Inhibition of BG on E. faecalis and C. albicans
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Volponi, A.A.; Zaugg, L.K.; Neves, V.; Liu, Y.; Sharpe, P.T. Tooth Repair and Regeneration. Curr. Oral Health Rep. 2018, 5, 295–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjørndal, L.; Simon, S.; Tomson, P.L.; Duncan, H.F. Management of deep caries and the exposed pulp. Int. Endod J. 2019, 52, 949–973. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.N.; Wang, Z.; Shen, Y.; Gavini, G.; Martinelli, J.R.; Manso, A.; Haapasalo, M. Comparative analyses of ion release, pH and multispecies biofilm formation between conventional and bioactive gutta-percha. Int. Endod. Endod. Endod. J. 2016, 49, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Zafar, M.S.; Amin, F.; Fareed, M.A.; Ghabbani, H.; Riaz, S.; Khurshid, Z.; Kumar, N. Biomimetic Aspects of Restorative Dentistry Biomaterials. Biomimetics 2020, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- AlRahabi, M.K. Predictors, prevention, and management of postoperative pain associated with nonsurgical root canal treatment: A systematic review. J. Taibah Univ. Med. Sci. 2017, 12, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Vianna, M.E.; Horz, H.P.; Conrads, G.; Zaia, A.A.; Souza-Filho, F.J.; Gomes, B.P.F.A. Effect of root canal procedures on endotoxins and endodontic pathogens. Oral Microbiol Immunol. 2007, 22, 411–418. [Google Scholar] [CrossRef]
- Vianna, M.E.; Gomes, B.P. Efficacy of sodium hypochlorite combined with chlorhexidine against Enterococcus faecalis in vitro. Oral Surg Oral Med. Oral Pathol Oral Radiol Endod. 2009, 107, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Sathorn, C.; Parashos, P.; Messer, H. Australian endodontists’ perceptions of single and multiple visit root canal treatment. Int. Endod J. 2009, 42, 811–818. [Google Scholar] [CrossRef]
- Attia, D.A.; Farag, A.M.; Afifi, I.K.; Darrag, A.M. Antimicrobial effect of different intracanal medications on various microorganisms. Tanta Dent. J. 2015, 12, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Elshinawy, M.I.; Al-Madboly, L.A.; Ghoneim, W.M.; El-Deeb, N.M. Synergistic Effect of Newly Introduced Root Canal Medicaments; Ozonated Olive Oil and Chitosan Nanoparticles, Against Persistent Endodontic Pathogens. Front. Microbiol. 2018, 9, 1371. [Google Scholar] [CrossRef] [Green Version]
- Suprewicz, Ł.; Tokajuk, G.; Cieśluk, M.; Deptuła, P.; Sierpińska, T.; Wolak, P.; Wollny, T.; Tokajuk, J.; Głuszek, S.; Piktel, E.; et al. Bacteria Residing at Root Canals Can Induce Cell Proliferation and Alter the Mechanical Properties of Gingival and Cancer Cells. Int. J. Mol. Sci. 2020, 21, 7914. [Google Scholar] [CrossRef] [PubMed]
- Divakar, N.; Mohan, S.P.; Pulyodan, M.K.; Tom, A.; Karukayil, D.; Somasundaram, M. Evaluation of Antimicrobial Efficacy of Calcium Hydroxide along with Proton Pump Inhibitor against Enterococcus Faecalis. J. Pharm Bioallied Sci. 2020, 12, S352–S354. [Google Scholar] [CrossRef] [PubMed]
- El-Telbany, M.; El-Didamony, G.; Askora, A.; Ariny, E.; Abdallah, D.; Connerton, I.F.; El-Shibiny, A. Bacteriophages to Control Multi-Drug Resistant Enterococcus faecalis Infection of Dental Root Canals. Microorganisms. 2021, 9, 517. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.A.; Rosa, V.; Min, K.S. Characterization of Enterococcus faecalis in different culture conditions. Sci. Rep. 2020, 10, 21867. [Google Scholar] [CrossRef]
- Alshanta, O.A.; Shaban, S.; Nile, C.J.; McLean, W.; Ramage, G. Candida albicans Biofilm Heterogeneity and Tolerance of Clinical Isolates: Implications for Secondary Endodontic Infections. Antibiotics 2019, 8, 204. [Google Scholar] [CrossRef] [Green Version]
- Yoo, Y.J.; Kim, A.R.; Perinpanayagam, H.; Han, S.H.; Kum, K.Y. Candida albicans Virulence Factors and Pathogenicity for Endodontic Infections. Microorganisms 2020, 8, 1300. [Google Scholar] [CrossRef]
- Rane, H.S.; Hayek, S.R.; Frye, J.E.; Abeyta, E.L.; Bernardo, S.M.; Parra, K.J.; Lee, S.A. Candida albicans Pma1p Contributes to Growth, pH Homeostasis, and Hyphal Formation. Front. Microbiol. 2019, 10, 1012. [Google Scholar] [CrossRef] [Green Version]
- Athanassiadis, B.; Abbott, P.V.; Walsh, L.J. The use of calcium hydroxide, antibiotics and biocides as antimicrobial medicaments in endodontics. Aust Dent. J. 2007, 52, S64–S82. [Google Scholar] [CrossRef]
- Carvalho, C.N.; Freire, L.G.; de Carvalho, A.P.; Luiz Siqueira, E.; Bauer, J.; Cunha Gritti, G.; Pereira de Souza, J.; Gavini, G. The Influence of Dentine on the pH of Calcium Hydroxide, Chlorhexidine Gel, and Experimental Bioactive Glass-Based Root Canal Medicament. Sci. World J. 2015, 2015, 686259. [Google Scholar] [CrossRef] [Green Version]
- Grazziotin-Soares, R.; Dourado, L.G.; Gonçalves, B.L.L.; Ardenghi, D.M.; Ferreira, M.C.; Bauer, J.; Carvalho, C.N. Dentin Microhardness and Sealer Bond Strength to Root Dentin are Affected by Using Bioactive Glasses as Intracanal Medication. Materiais 2020, 13, 721. [Google Scholar] [CrossRef] [Green Version]
- Kahler, S.L.; Shetty, S.; Andreasen, F.M.; Kahler, B. The Effect of Long-term Dressing with Calcium Hydroxide on the Fracture Susceptibility of Teeth. J. Endodontics 2018, 44, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Pabel, A.K.; Hülsmann, M. Comparison of different techniques for removal of calcium hydroxide from straight root canals: An in vitro study. Odontology 2017, 105, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.; Manjunath, M.K.; Tejaswi, S. An In-vitro Evaluation of the pH Change Through Root Dentin Using Different Cal-cium Hydroxide Preparations as an Intracanal Medicament. J. Clin. Diagn Res. 2014, 8, ZC13–ZC16. [Google Scholar] [CrossRef]
- Fernandes, H.R.; Gaddam, A.; Rebelo, A.; Brazete, D.; Stan, G.E.; Ferreira, J.M.F. Bioactive Glasses and Glass-Ceramics for Healthcare Applications in Bone Regeneration and Tissue Engineering. Materials 2018, 11, 2530. [Google Scholar] [CrossRef] [Green Version]
- Sergi, R.; Bellucci, D.; Cannillo, V. A Review of Bioactive Glass/Natural Polymer Composites: State of the Art. Materials 2020, 13, 5560. [Google Scholar] [CrossRef]
- Gonzalez, M.M.; Butini, M.E.; Maiolo, E.M.; Sessa, L.; Trampuz, A. Antimicrobial activity of bioactive glass S53P4 against representative microorganisms causing osteomyelitis—Real-time assessment by isothermal microcalorimetry. Colloids Surf. B Biointerfaces 2020, 189, 110853. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, M.; Söderling, E.; Salonen, J.; Waltimo, T. Preliminary evaluation of bioactive glass S53P4 as an endodontic medication in vitro. J. Endod. 2004, 30, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Swe, T.T.; Mohamad, H.; Shariff, K.A.; Ishikawa, K. Synthesis and Characterization of Sol-Gel Derived Strontium Doped S53P4 Bioglass. In Key Eng. Mat. 2022, 908, 141–147. [Google Scholar] [CrossRef]
- Tite, T.; Popa, A.C.; Stuart, B.W.; Fernandes, H.R.; Chirica, I.M.; Lungu, G.A.; Macovei, D.; Bartha, C.; Albulescu, L.; Tanase, C.; et al. Independent and complementary bio-functional effects of CuO and Ga2O3 incorporated as therapeutic agents in silica and phosphate-based bioactive glasses. J. Materiomics, 2022; in press. [Google Scholar] [CrossRef]
- Liu, J.; Rawlinson, S.C.; Hill, R.G.; Fortune, F. Strontium-substituted bioactive glasses in vitro osteogenic and antibacterial effects. Dent. Mater. 2016, 32, 412–422. [Google Scholar] [CrossRef]
- Fiume, E.; Barberi, J.; Verné, E.; Baino, F. Bioactive Glasses: From Parent 45S5 Composition to Scaffold-Assisted Tissue-Healing Therapies. J. Funct Biomater. 2018, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Bento, R.; Gaddam, A.; Oskoei, P.; Oliveira, H.; Ferreira, J.M.F. 3D Printing of Macro Porous Sol-Gel Derived Bioactive Glass Scaffolds and Assessment of Biological Response. Materials 2021, 14, 5946. [Google Scholar] [CrossRef] [PubMed]
- Ben-Arfa, B.A.E.; Salvado, I.M.M.; Ferreira, J.M.F.; Pullar, R.C. A hundred times faster: Novel, rapid sol-gel synthesis of bio-glass nanopowders (Si-Na-Ca-P system, Ca:P = 1.67) without aging. Int. J. Appl. Glass Sci. 2016, 8, 337–343. [Google Scholar] [CrossRef]
- Ben-Arfa, B.A.E.; Salvado, I.M.M.; Pullar, R.C.; Ferreira, J.M.F. The influence of processing parameters on morphology and granulometry of a wet-milled sol-gel glass powder. Ceram. Int. 2018, 44, 12754–12762. [Google Scholar] [CrossRef]
- Ben-Arfa, B.A.E.; Salvado, I.M.M.; Frade, J.R.; Pullar, R.C. Guidelines to adjust particle size distributions by wet comminution of a bio-active glass determined by taguchi and multivariate analysis. Ceram. Int. 2019, 45, 3857–3863. [Google Scholar] [CrossRef]
- Naghili, H.; Tajik, H.; Mardani, K.; Razavi Rouhani, S.M.; Ehsani, A.; Zare, P. Validation of drop plate technique for bacterial enumeration by parametric and nonparametric tests. Vet. Res. Forum. 2013, 4, 179–183. [Google Scholar]
- Dean, J.A. Lange’s Handbook of Chemistry, 15th ed.; McGraw-Hill: New York, NY, USA, 1999; p. 1424. [Google Scholar]
- Gaddam, A.; Gołębiewski, P.; Fernandes, H.R.; Pysz, D.; Neto, A.S.; Diduszko, R.; Malinowska, A.; Stępień, R.; Cimek, J.; Buczyński, R.; et al. Development of microfibers for bone regeneration based on alkali-free bioactive glasses doped with boron oxide. Am. Ceram. Soc. 2021, 104, 4492–4504. [Google Scholar] [CrossRef]
- DeCapitani, C.; Kirschen, M. A generalized multicomponent excess function with application to immiscible liquids in the system CaO-SiO2-TiO2. Geochim. Cosmochim. Acta 1998, 62, 3753–3763. [Google Scholar] [CrossRef]
- Souza, G.P.; Fokin, V.M.; Rodrigues, C.F.; Rodrigues, A.C.M.; Zanotto, E.D.; Lumeau, J.; Glebova, L.; Glebov, L.B. Liquid–Liquid Phase Separation in Photo-Thermo-Refractive Glass. Am. Ceram. Soc. 2010, 94, 145–150. [Google Scholar] [CrossRef]
- Dziadek, M.; Zagrajczuk, B.; Jelen, P.; Olejniczak, Z.; Cholewa-Kowalska, K. Structural variations of bioactive glasses obtained by different synthesis routes. Ceram. Int. 2016, 42, 14700–14709. [Google Scholar] [CrossRef]
- Aguiar, H.; Serra, J.; González, P.; León, B. Structural study of sol–gel silicate glasses by IR and Raman spectroscopies. J. Non Cryst. Solids 2009, 355, 475–480. [Google Scholar] [CrossRef]
- Schlumberger, C.; Thommes, M. Characterization of Hierarchically Ordered Porous Materials by Physisorption and Mercury Porosimetry—A Tutorial Review. Adv. Mater. Interfaces 2021, 8, 2002181. [Google Scholar] [CrossRef]
- Drago, L.; Toscano, M.; Bottagisio, M. Recent Evidence on Bioactive Glass Antimicrobial and Antibiofilm Activity: A Mini-Review. Materials 2018, 11, 326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pankey, G.A.; Sabath, L.D. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin. Infect. Dis. 2004, 38, 864–870. [Google Scholar] [CrossRef] [Green Version]
- Leppäranta, O.; Vaahtio, M.; Peltola, T.; Zhang, D.; Hupa, L.; Hupa, M.; Ylänen, H.; Salonen, J.I.; Viljanen, M.K.; Eerola, E. Antibacterial effect of bioactive glasses on clinically important anaerobic bacteria in vitro. J. Mater. Sci. Mater. Med. 2008, 19, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Munukka, E.; Leppäranta, O.; Korkeamäki, M.; Vaahtio, M.; Peltola, T.; Zhang, D.; Hupa, L.; Ylänen, H.; Salonen, J.I.; Viljanen, M.K.; et al. Bactericidal effects of bioactive glasses on clinically important aerobic bacteria. J. Mater. Sci. Mater. Med. 2008, 19, 27–32. [Google Scholar] [CrossRef]
- Zhang, D.; Leppäranta, O.; Munukka, E.; Ylänen, H.; Viljanen, M.K.; Eerola, E.; Hupa, M.; Hupa, L. Antibacterial effects and dissolution behavior of six bioactive glasses. J. Biomed. Mater. Res. A 2010, 93, 475–483. [Google Scholar] [CrossRef]
- Popa, A.C.; Fernandes, H.R.; Necsulescu, M.; Luculescu, C.; Cioangher, M.; Dumitru, V.; Stuart, B.W.; Grant, D.M.; Ferreira, J.M.F.; Stan, G.E. Antibacterial efficiency of alkali-free bio-glasses incorporating ZnO and/or SrO as therapeutic agents. Cer. Int. 2019, 45, 4368–4380. [Google Scholar] [CrossRef]
- Singh, S.; Patil, A.; Mali, S.; Jaiswal, H. Bioglass: A New Era in Modern Dentistry. Eur. J. Dent. 2022, 11, 001–006. [Google Scholar] [CrossRef]
- Goel, A.; Sinha, A.; Khandeparker, R.V.; Mehrotra, R.; Vashisth, P.; Garg, A. Bioactive Glass S53P4 versus Chlorhexidine Gluconate as Intracanal Medicament in Primary Teeth: An In-vivo Study Using Polymerase Chain Reaction Analysis. J. Int. Oral Health 2015, 7, 65–69. [Google Scholar]
- Ranga, N.; Gahlyan, S.; Duhan, S. Antibacterial Efficiency of Zn, Mg and Sr Doped Bioactive Glass for Bone Tissue Engineering. J. NanoSci. Nanotechnol. 2020, 20, 2465–2472. [Google Scholar] [CrossRef]
- Stan, G.E.; Popa, A.C.; Chirica, I.M.; Negrila, C.C.; Besleaga, C.; Zgura, I.; Sergentu, A.C.; Popescu-Pelin, G.; Cristea, D.; Ionescu, L.E.; et al. The Beneficial Mechanical and Biological Outcomes of Thin Copper-Gallium Doped Silica-Rich Bio-Active Glass Implant-Type Coatings. Coatings 2020, 10, 1119. [Google Scholar] [CrossRef]







| Molar % | |||||||
|---|---|---|---|---|---|---|---|
| Bioactive Glass | SiO2 | CaO | MgO | SrO | ZnO | CuO | P2O5 |
| BG1 | 50 | 20 | 10 | 10 | 6 | 2 | 2 |
| BG2 | 50 | 20 | 5 | 15 | 6 | 2 | 2 |
| BG3 | 50 | 15 | 5 | 15 | 10 | 3 | 2 |
| Bioactive Glass | D10 (μm) | D50 (μm) | D90 (μm) | Average Particle Size (μm) | Standard Deviation (μm) |
|---|---|---|---|---|---|
| BG1 | 0.87 | 2.87 | 7.86 | 3.70 | 2.86 |
| BG2 | 0.85 | 2.62 | 5.87 | 3.03 | 1.96 |
| BG3 | 0.86 | 2.36 | 4.78 | 2.62 | 1.52 |
| pH of Candida albicans Suspensions | pH of Enterococcus faecalis Suspensions | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Samples and Concentrations (mg mL−1) | Time 0 h | Time 24 h | Time 48 h | Samples and Concentrations (mg mL−1) | Time 0 h | Time 24 h | Time 48 h | ||
| BG1 | 5 | 9.53 | 6.94 | 6.92 | BG1 | 5 | 8.12 | 7.58 | 7.67 |
| 15 | 10.20 | 10.04 | 9.79 | 15 | 9.23 | 9.05 | 9.06 | ||
| BG2 | 5 | 9.56 | 6.88 | 6.85 | BG2 | 5 | 9.03 | 7.80 | 7.79 |
| 15 | 10.53 | 9.93 | 9.87 | 15 | 9.13 | 8.93 | 8.85 | ||
| BG3 | 5 | 9.74 | 6.58 | 6.89 | BG3 | 5 | 8.17 | 7.36 | 7.47 |
| 15 | 10.79 | 10.25 | 10.21 | 15 | 9.12 | 9.06 | 9.02 | ||
| C. albicans control | 5.61 | 4.56 | 4.41 | E. faecalis control | 5.34 | 4.99 | 5.17 | ||
| SDB | 6.26 | 6.26 | 6.26 | BHI | 7.16 | 7.16 | 7.16 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Correia, B.L.; Gomes, A.T.P.C.; Noites, R.; Ferreira, J.M.F.; Duarte, A.S. New and Efficient Bioactive Glass Compositions for Controlling Endodontic Pathogens. Nanomaterials 2022, 12, 1577. https://doi.org/10.3390/nano12091577
Correia BL, Gomes ATPC, Noites R, Ferreira JMF, Duarte AS. New and Efficient Bioactive Glass Compositions for Controlling Endodontic Pathogens. Nanomaterials. 2022; 12(9):1577. https://doi.org/10.3390/nano12091577
Chicago/Turabian StyleCorreia, Bruna L., Ana T. P. C. Gomes, Rita Noites, José M. F. Ferreira, and Ana S. Duarte. 2022. "New and Efficient Bioactive Glass Compositions for Controlling Endodontic Pathogens" Nanomaterials 12, no. 9: 1577. https://doi.org/10.3390/nano12091577
APA StyleCorreia, B. L., Gomes, A. T. P. C., Noites, R., Ferreira, J. M. F., & Duarte, A. S. (2022). New and Efficient Bioactive Glass Compositions for Controlling Endodontic Pathogens. Nanomaterials, 12(9), 1577. https://doi.org/10.3390/nano12091577