The Effect of Agglomeration on Arsenic Adsorption Using Iron Oxide Nanoparticles
Abstract
:1. Introduction
2. Methods
2.1. Materials
2.1.1. Synthesis and Characterization of IONPs
2.1.2. Arsenic Adsorption Experiments
2.1.3. Column Experiments
2.2. Rapid Small Scale Column Tests
2.2.1. Packing Solid Nanoparticles
2.2.2. Packing Nanoparticles Synthesized via Thermal Decomposition
2.3. Batch Experiments for Arsenic
2.3.1. Adsorption Isotherms Using Solids
2.3.2. Adsorption Isotherms Using IONP Solutions
3. Results and Discussion
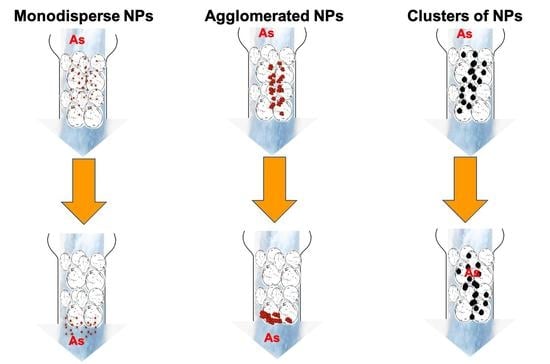
3.1. Effect of Agglomeration of IONPs Deposited onto Sand in Column Experiments
3.2. Usage of Clusters of IONPs in Column Experiments
3.3. Comparison of Arsenic Adsorption on a Batch Setup
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arsenic. Available online: https://www.who.int/news-room/fact-sheets/detail/arsenic (accessed on 27 April 2022).
- Mandal, B.K.; Suzuki, K.T. Arsenic Round the World: A Review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Mondal, P.; Bhowmick, S.; Chatterjee, D.; Figoli, A.; Van der Bruggen, B. Remediation of Inorganic Arsenic in Groundwater for Safe Water Supply: A Critical Assessment of Technological Solutions. Chemosphere 2013, 92, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Liu, M.; Wang, N.; Li, G. A Critical Review on Arsenic Removal from Water Using Iron-Based Adsorbents. RSC Adv. 2018, 8, 39545–39560. [Google Scholar] [CrossRef]
- Li, S.; Guo, Y.; Xiao, M.; Zhang, T.; Yao, S.; Zang, S.; Fan, H.; Shen, Y.; Zhang, Z.; Li, W. Enhanced Arsenate Removal from Aqueous Solution by Mn-Doped MgAl-Layered Double Hydroxides. Environ. Sci. Pollut. Res. 2019, 26, 12014–12024. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy Metal Removal from Water/Wastewater by Nanosized Metal Oxides: A Review. J. Hazard. Mater. 2012, 211–212, 317–331. [Google Scholar] [CrossRef]
- Lee, L.Z.; Zaini, M.A.A.; Tang, S.H. Porous nanomaterials for heavy metal removal. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 469–494. [Google Scholar] [CrossRef]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric Membranes Incorporated with Metal/Metal Oxide Nanoparticles: A Comprehensive Review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Modrogan, C.; Cǎprǎrescu, S.; Dǎncilǎ, A.M.; Orbuleț, O.D.; Vasile, E.; Purcar, V. Mixed Oxide Layered Double Hydroxide Materials: Synthesis, Characterization and Efficient Application for Mn2+ Removal from Synthetic Wastewater. Materials 2020, 13, 4089. [Google Scholar] [CrossRef]
- Kubacka, A.; Fernández-García, M.; Colón, G. Advanced Nanoarchitectures for Solar Photocatalytic Applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar] [CrossRef]
- Liang, M.; Guo, L.-H. Application of Nanomaterials in Environmental Analysis and Monitoring. J. Nanosci. Nanotechnol. 2009, 9, 2283–2289. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Matis, K.A. Nanoadsorbents for Pollutants Removal: A Review. J. Mol. Liq. 2015, 203, 159–168. [Google Scholar] [CrossRef]
- Gómez-Pastora, J.; Bringas, E.; Ortiz, I. Recent Progress and Future Challenges on the Use of High Performance Magnetic Nano-Adsorbents in Environmental Applications. Chem. Eng. J. 2014, 256, 187–204. [Google Scholar] [CrossRef]
- Modrogan, C.; Cǎprǎrescu, S.; Dǎncilǎ, A.M.; Orbuleț, O.D.; Grumezescu, A.M.; Purcar, V.; Radițoiu, V.; Fierascu, R.C. Modified Composite Based on Magnetite and Polyvinyl Alcohol: Synthesis, Characterization, and Degradation Studies of the Methyl Orange Dye from Synthetic Wastewater. Polymers 2021, 13, 3911. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Hernández, M.; Arrieta, R.A.; Ventura, K.; Hernández, J.; Powell, C.D.; Atkinson, A.J.; Markovski, J.S.; Gardea-Torresdey, J.; Hristovski, K.D.; Westerhoff, P.; et al. Superparamagnetic Nanoadsorbents for the Removal of Trace As(III) in Drinking Water. Environ. Adv. 2021, 4, 100046. [Google Scholar] [CrossRef]
- Nigam, B.; Mittal, S.; Prakash, A.; Satsangi, S.; Mahto, P.K.; Swain, B.P. Synthesis and Characterization of Fe3O4 Nanoparticles for Nanofluid Applications-A Review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 377, 012187. [Google Scholar] [CrossRef]
- Sanna Angotzi, M.; Mameli, V.; Fantasia, A.; Cara, C.; Secci, F.; Enzo, S.; Gerina, M.; Cannas, C. As(III, V) Uptake from Nanostructured Iron Oxides and Oxyhydroxides: The Complex Interplay between Sorbent Surface Chemistry and Arsenic Equilibria. Nanomaterials 2022, 12, 326. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, E.; Oldham, T.; Li, W.; Fortner, J. Chapter 5: Tuning iron oxide-based nanomaterials as next generation adsorbents for environmental applications. In Emerging Nanotechnologies for Water Treatment; Elsevier: Amsterdam, The Netherlands, 2021; pp. 117–152. [Google Scholar] [CrossRef]
- Jain, A.; Raven, K.P.; Loeppert, R.H. Arsenite and Arsenate Adsorption on Ferrihydrite: Surface Charge Reduction and Net OH- Release Stoichiometry. Environ. Sci. Technol. 1999, 33, 1179–1184. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, S.-B.; Lee, S.-H.; Choi, J.-W. Laboratory and Pilot-Scale Field Experiments for Application of Iron Oxide Nanoparticle-Loaded Chitosan Composites to Phosphate Removal from Natural Water. Environ. Technol. 2018, 39, 770–779. [Google Scholar] [CrossRef]
- Farrell, J.W.; Fortner, J.; Work, S.; Avendano, C.; Gonzalez-Pech, N.I.; Zárate Araiza, R.; Li, Q.; Álvarez, P.J.J.; Colvin, V.; Kan, A.; et al. Arsenic Removal by Nanoscale Magnetite in Guanajuato, Mexico. Environ. Eng. Sci. 2014, 31, 393–402. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Pech, N.I.; Molloy, A.L.; Zambrano, A.; Lin, W.; Bohloul, A.; Zarate-Araiza, R.; Avendano, C.; Colvin, V.L. Feasibility of Iron-based Sorbents for Arsenic Removal from Groundwater. J. Chem. Tech. Biotech. 2022, jctb.7057. [Google Scholar] [CrossRef]
- Yean, S.; Cong, L.; Yavuz, C.T.; Mayo, J.T.; Yu, W.W.; Kan, A.T.; Colvin, V.L.; Tomson, M.B. Effect of Magnetite Particle Size on Adsorption and Desorption of Arsenite and Arsenate. J. Mater. Res. 2005, 20, 3255–3264. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Peng, W.; Zare, Y.; Rhee, K.Y. Effects of Size and Aggregation/Agglomeration of Nanoparticles on the Interfacial/Interphase Properties and Tensile Strength of Polymer Nanocomposites. Nanoscale Res. Lett. 2018, 13, 214. [Google Scholar] [CrossRef] [PubMed]
- Molloy, A.L.; Andrade, M.F.C.; Escalera, G.; Bohloul, A.; Avendano, C.; Colvin, V.L.; Gonzalez-Pech, N.I. The Effect of Surface Coating on Iron-Oxide Nanoparticle Arsenic Adsorption. MRS Adv. 2021, 6, 867–874. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, L.; Wang, H.; Yang, W.; Fu, Y.; Zhou, W.; Yu, W.; Xiang, K.; Su, Z.; Dai, S.; et al. Controllable Synthesis of Hierarchical Porous Fe 3 O 4 Particles Mediated by Poly(Diallyldimethylammonium Chloride) and Their Application in Arsenic Removal. ACS Appl. Mater. Interfaces 2013, 5, 12449–12459. [Google Scholar] [CrossRef] [PubMed]
- Ogata, A.; Banks, R.B. A Solution of the Differential Equation of Longitudinal Dispersion in Porous Media; Professional Paper; United States Department of the Interior: Washington, DC, USA, 1961. [Google Scholar]
- Xiao, Z.; Zhang, L.; Colvin, V.L.; Zhang, Q.; Bao, G. Synthesis and Application of Magnetic Nanocrystal Clusters. Ind. Eng. Chem. Res. 2022. [Google Scholar] [CrossRef]
- Watson, J.S. Simplified Predictions of Breakthrough Fronts for Constant-Pattern Adsorption and Ion Exchange. Sep. Sci. Technol. 1995, 30, 1351–1371. [Google Scholar] [CrossRef]
- US EPA, O. National Primary Drinking Water Regulations. Available online: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations (accessed on 27 March 2022).
- Sun, X.; Hu, C.; Hu, X.; Qu, J.; Yang, M. Characterization and Adsorption Performance of Zr-Doped Akaganéite for Efficient Arsenic Removal. J. Chem. Technol. Biotechnol. 2013, 88, 629–635. [Google Scholar] [CrossRef]
- Yu, W.W.; Falkner, J.C.; Yavuz, C.T.; Colvin, V.L. Synthesis of Monodisperse Iron Oxide Nanocrystals by Thermal Decomposition of Iron Carboxylate Salts. Chem. Commun. 2004, 2306–2307. [Google Scholar] [CrossRef]
- Park, J.; An, K.; Hwang, Y.; Park, J.-G.; Noh, H.-J.; Kim, J.-Y.; Park, J.-H.; Hwang, N.-M.; Hyeon, T. Ultra-Large-Scale Syntheses of Monodisperse Nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef]
- Yu, D.; Sun, X.; Zou, J.; Wang, Z.; Wang, F.; Tang, K. Oriented Assembly of Fe3O4 Nanoparticles into Monodisperse Hollow Single-Crystal Microspheres. J. Phys. Chem. B 2006, 110, 21667–21671. [Google Scholar] [CrossRef]






| L (cm) | BV (mL) | Q (mL/min) | R | D (cm2/min) | r2 | |
|---|---|---|---|---|---|---|
| Thermal decomposition | 7 | 5.5 | 2.8 | 19 | 6.3 | 0.976 |
| Coprecipitation | 10.5 | 8.3 | 4.1 | 145 | 31.8 | 0.990 |
| Commercial | 25.5 | 20.0 | 6.4 | 13 | 73.3 | 0.961 |
| Clusters of Nanoparticles | 3.5 | 2.8 | 1.4 | 180 | 1.8 | 0.856 |
| qmax (mg/g) | kL (L/mg) | q10 (mg/g) | q100 (mg/g) | |
|---|---|---|---|---|
| Thermal decomposition | 11.1 | 0.6 | 0.1 | 0.7 |
| Coprecipitation | 6.6 | 1.8 | 0.1 | 1.0 |
| Commercial | 0.6 | 31.4 | 0.1 | 0.5 |
| Clusters of Nanoparticles | 121.4 | 1.4 | 1.6 | 14.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diephuis, W.R.; Molloy, A.L.; Boltz, L.L.; Porter, T.B.; Aragon Orozco, A.; Duron, R.; Crespo, D.; George, L.J.; Reiffer, A.D.; Escalera, G.; et al. The Effect of Agglomeration on Arsenic Adsorption Using Iron Oxide Nanoparticles. Nanomaterials 2022, 12, 1598. https://doi.org/10.3390/nano12091598
Diephuis WR, Molloy AL, Boltz LL, Porter TB, Aragon Orozco A, Duron R, Crespo D, George LJ, Reiffer AD, Escalera G, et al. The Effect of Agglomeration on Arsenic Adsorption Using Iron Oxide Nanoparticles. Nanomaterials. 2022; 12(9):1598. https://doi.org/10.3390/nano12091598
Chicago/Turabian StyleDiephuis, William R., Anna L. Molloy, Lindsey L. Boltz, Tristan B. Porter, Anthony Aragon Orozco, Reina Duron, Destiny Crespo, Luke J. George, Andrew D. Reiffer, Gabriela Escalera, and et al. 2022. "The Effect of Agglomeration on Arsenic Adsorption Using Iron Oxide Nanoparticles" Nanomaterials 12, no. 9: 1598. https://doi.org/10.3390/nano12091598
APA StyleDiephuis, W. R., Molloy, A. L., Boltz, L. L., Porter, T. B., Aragon Orozco, A., Duron, R., Crespo, D., George, L. J., Reiffer, A. D., Escalera, G., Bohloul, A., Avendano, C., Colvin, V. L., & Gonzalez-Pech, N. I. (2022). The Effect of Agglomeration on Arsenic Adsorption Using Iron Oxide Nanoparticles. Nanomaterials, 12(9), 1598. https://doi.org/10.3390/nano12091598