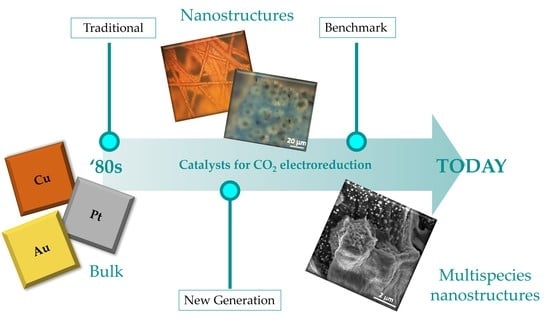
From Traditional to New Benchmark Catalysts for CO2 Electroreduction
Abstract
:1. Introduction
2. Electrochemical CO2 Conversion
2.1. Traditional Electrocatalysts
2.2. Electrocatalysts of the New Generation
2.3. Benchmark Electrocatalysts
3. Conclusions and Future Perspectives
- (i)
- Even if a growing number of reports concerning the local investigation of the electrode/electrolyte interface are being released using in situ and operando techniques, the thorough understanding of reaction mechanisms has not been fulfilled yet. Improved knowledge about local phenomena occurring on the nano- and microscale would be greatly beneficial for the optimisation of the catalyst composition and morphological properties depending on the experimental conditions of choice. This should impact the overall, macroscopic, electrochemical performances, for instance, lowering the required overpotential, enhancing the productivity towards the desired species, and improving the catalyst stability and regeneration capability. To this end, the interplay among different interfacial techniques as well as computational modelling is highly encouraged.
- (ii)
- CO2ER systems are often investigated regardless of the anodic part of the electrochemical cell. Not only should the anode not be ignored due to its impact on the overall cost and sustainability of the final device, but it could also be exploited to combine the conversion of CO2 with another valuable electrochemical reaction, such as oxygen evolution or wastewater treatment. From this point of view, the design of versatile, multi-functional electrocatalysts would represent a further improvement in the overall efficiency of the cell.
- (iii)
- Different from other electrochemical processes for energy storage and production, no standardised protocol or benchmark has been established for the electrochemical reduction of CO2 yet [165]. The development of such tools would help the objective evaluation and comparison of electrocatalysts’ performances.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karl, T.R.; Trenberth, K.E. Modern Global Climate Change. Science 2003, 302, 1719–1723. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, P.G.; Rigby, M.; Manning, A.J.; Park, S.; Stanley, K.M.; McCulloch, A.; Henne, S.; Graziosi, F.; Maione, M.; Arduini, J.; et al. The increasing atmospheric burden of the greenhouse gas sulfur hexafluoride (SF6). Atmos. Chem. Phys. 2020, 20, 7271–7290. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. IPCC, 2013: Summary for Policymakers. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Intergovernmental Panel on Climate Change: Cambridge, UK; New York, NY, USA, 2013; pp. 3–303. Available online: https://www.ipcc.ch/site/assets/uploads/2018/03/WG1AR5_SummaryVolume_FINAL.pdf (accessed on 9 September 2022).
- Haibach, H.; Schneider, K. The Politics of Climate Change: Review and Future Challenges. In Climate Change: International Law and Global Governance, 1st ed.; Ruppel, O.C., Roschmann, C., Ruppel-Schlichting, K., Eds.; Nomos Verlagsgesellschaft mbH: Baden-Baden, Germany, 2013; Volume 2, pp. 357–374. Available online: http://www.jstor.org/stable/j.ctv941vsk.18 (accessed on 3 September 2022).
- Zillman, J.W. A History of Climate Activities. 2009, pp. 141–150. Available online: https://public.wmo.int/en/bulletin/history-climate-activities (accessed on 3 September 2022).
- Farman, J.C.; Gardiner, B.G.; Shanklin, J.D. Large Losses of Total Ozone in Antarctica Reveal Seasonal ClOx/NOx Interaction. Nature 1985, 315, 207–210. [Google Scholar] [CrossRef]
- Solomon, S. The Discovery of the Antarctic Ozone Hole. Nature 2019, 575, 46–47. [Google Scholar] [CrossRef] [PubMed]
- Houghton, J.T.; Jenkins, G.J.; Ephraums, J.J. Climate Change: The IPCC Scientific Assessment; IPCC 1990; Cambridge University Press: Cambridge, UK; New York, NY, USA; Melbourne, Australia, 1990; pp. 1–410. Available online: https://archive.ipcc.ch/publications_and_data/publications_ipcc_first_assessment_1990_wg1.shtml (accessed on 3 September 2022).
- Gupta, J. A History of International Climate Change Policy. WIREs Clim. Chang. 2010, 1, 636–653. [Google Scholar] [CrossRef]
- Aichele, R.; Felbermayr, G. The Effect of the Kyoto Protocol on Carbon Emissions. J. Policy Anal. Manag. 2013, 32, 731–757. [Google Scholar] [CrossRef]
- EEA. Report 18/2014—Progress towards 2008–2012 Kyoto Targets in Europe; EEA: Luxembourg, 2014; pp. 1–62. Available online: https://www.eea.europa.eu/publications/progress-towards-2008-2012-kyoto (accessed on 3 September 2022).
- Rosen, A.M. The Wrong Solution at the Right Time: The Failure of the Kyoto Protocol on Climate Change. Polit. Policy 2015, 43, 30–58. [Google Scholar] [CrossRef]
- Kumazawa, R.; Callaghan, M.S. The effect of the Kyoto Protocol on carbon dioxide emissions. J. Econ. Financ. 2012, 36, 201–210. [Google Scholar] [CrossRef]
- Almer, C.; Winkler, R. Analyzing the effectiveness of international environmental policies: The case of the Kyoto Protocol. J. Environ. Econ. Manag. 2017, 82, 125–151. [Google Scholar] [CrossRef]
- Helm, D. The Kyoto approach has failed. Nature 2012, 491, 663–665. [Google Scholar] [CrossRef]
- Grunewald, N.; Martinez-Zarzoso, I. Did the Kyoto Protocol fail? An evaluation of the effect of the Kyoto Protocol on CO2 emissions. Environ. Dev. Econ. 2016, 21, 1–22. [Google Scholar] [CrossRef]
- Larsen, K.; Pitt, H.; Grant, M.; Houser, T. China’s Greenhouse Gas Emissions Exceeded the Developed World for the First Time in 2019. Available online: https://rhg.com/research/chinas-emissions-surpass-developed-countries/#_ftn1 (accessed on 3 September 2022).
- Falkner, R. The Paris Agreement and the New Logic of International Climate Politics. Int. Aff. 2016, 92, 1107–1125. [Google Scholar] [CrossRef]
- The Royal Society. Dealing with Carbon Dioxide at Scale; The Royal Society: London, UK, 2017; pp. 1–22. Available online: http://www.nasonline.org/programs/scientific-forum/sackler-forum-2017-carbon.pdf (accessed on 18 August 2022).
- Morrow, D.R.; Thompson, M.S. Reduce, Remove, Recycle: Clarifying the Overlap between Carbon Removal and CCUS; Institute for Carbon Removal Law and Policy, American University: Washington, DC, USA, 2020; pp. 1–11. Available online: http://research.american.edu/carbonremoval/wp-content/uploads/sites/3/2020/12/reduce-remove-recycle_final.pdf (accessed on 18 August 2022).
- Roy, S.; Cherevotan, A.; Peter, S.C. Thermochemical CO2 Hydrogenation to Single Carbon Products: Scientific and Technological Challenges. ACS Energy Lett. 2018, 3, 1938–1966. [Google Scholar] [CrossRef]
- Shi, J.; Jiang, Y.; Jiang, Z.; Wang, X.; Wang, X.; Zhang, S.; Han, P.; Yang, C. Enzymatic conversion of carbon dioxide. Chem. Soc. Rev. 2015, 44, 5981–6000. [Google Scholar] [CrossRef]
- Sultana, S.; Chandra Sahoo, P.; Martha, S.; Parida, K. A review of harvesting clean fuels from enzymatic CO2 reduction. RSC Adv. 2016, 6, 44170–44194. [Google Scholar] [CrossRef]
- Habisreutinger, S.N.; Schmidt-Mende, L.; Stolarczyk, J.K. Photocatalytic Reduction of CO2 on TiO2 and Other Semiconductors. Angew. Chem. Int. Ed. 2013, 52, 7372–7408. [Google Scholar] [CrossRef] [PubMed]
- Rej, S.; Bisetto, M.; Naldoni, A.; Fornasiero, P. Well-defined Cu2O photocatalysts for solar fuels and chemicals. J. Mater. Chem. A 2021, 9, 5915–5951. [Google Scholar] [CrossRef]
- Genovese, C.; Ampelli, C.; Perathoner, S.; Centi, G. Electrocatalytic conversion of CO2 to liquid fuels using nanocarbon-based electrodes. J. Energy Chem. 2013, 22, 202–213. [Google Scholar] [CrossRef]
- Serafini, M.; Mariani, F.; Fasolini, A.; Scavetta, E.; Basile, F.; Tonelli, D. Nanostructured Copper-Based Electrodes Electrochemically Synthesized on a Carbonaceous Gas Diffusion Membrane with Catalytic Activity for the Electroreduction of CO2. ACS Appl. Mater. Interfaces 2021, 13, 57451–57461. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. Catalytic Thermal Conversion of CO2 into Fuels: Perspective and Challenges. Renew. Sustain. Energy Rev. 2019, 115, 1–20. [Google Scholar] [CrossRef]
- Marlin, D.S.; Sarron, E.; Sigurbjörnsson, Ó. Process Advantages of Direct CO2 to Methanol Synthesis. Front. Chem. 2018, 6, 446. [Google Scholar] [CrossRef] [PubMed]
- Yaashikaa, P.R.; Senthil Kumar, P.; Varjani, S.J.; Saravanan, A. A Review on Photochemical, Biochemical and Electrochemical Transformation of CO2 into Value-Added Products. J. CO2 Util. 2019, 33, 131–147. [Google Scholar] [CrossRef]
- Gupta, R.; Mishra, A.; Thirupathaiah, Y.; Chandel, A.K. Biochemical Conversion of CO2 in Fuels and Chemicals: Status, Innovation, and Industrial Aspects. Biomass Convers. Biorefinery 2022, 1–24. [Google Scholar] [CrossRef]
- Gao, Y.; Qian, K.; Xu, B.; Li, Z.; Zheng, J.; Zhao, S.; Ding, F.; Sun, Y.; Xu, Z. Recent Advances in Visible-Light-Driven Conversion of CO2 by Photocatalysts into Fuels or Value-Added Chemicals. Carbon Resour. Convers. 2020, 3, 46–59. [Google Scholar] [CrossRef]
- Garg, S.; Li, M.; Weber, A.Z.; Ge, L.; Li, L.; Rudolph, V.; Wang, G.; Rufford, T.E. Advances and Challenges in Electrochemical CO2 Reduction Processes: An Engineering and Design Perspective Looking beyond New Catalyst Materials. J. Mater. Chem. A 2020, 8, 1511–1544. [Google Scholar] [CrossRef]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef]
- She, X.; Wang, Y.; Xu, H.; Chi Edman Tsang, S.; Ping Lau, S. Challenges and Opportunities in Electrocatalytic CO2 Reduction to Chemicals and Fuels. Angew. Chemie Int. Ed. 2022, 61, e202211396. [Google Scholar] [CrossRef]
- Agarwal, A.S.; Zhai, Y.; Hill, D.; Sridhar, N. Corrigendum: The Electrochemical Reduction of Carbon Dioxide to Formate/Formic Acid: Engineering and Economic Feasibility. ChemSusChem 2011, 4, 1705. [Google Scholar] [CrossRef]
- Bevilacqua, M.; Filippi, J.; Miller, H.A.; Vizza, F. Recent Technological Progress in CO2 Electroreduction to Fuels and Energy Carriers in Aqueous Environments. Energy Technol. 2015, 3, 197–210. [Google Scholar] [CrossRef]
- Li, L.; Huang, Y.; Li, Y. Carbonaceous materials for electrochemical CO2 reduction. EnergyChem 2020, 2, 100024. [Google Scholar] [CrossRef]
- Jin, S.; Hao, Z.; Zhang, K.; Yan, Z.; Chen, J. Advances and Challenges for the Electrochemical Reduction of CO2 to CO: From Fundamentals to Industrialization. Angew. Chem. Int. Ed. 2021, 60, 20627–20648. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Hasija, V.; Sudhaik, A.; Raizada, P.; Van Le, Q.; Singh, P.; Pham, T.-H.; Kim, T.; Ghotekar, S.; Nguyen, V.-H. Artificial leaf for light-driven CO2 reduction: Basic concepts, advanced structures and selective solar-to-chemical products. Chem. Eng. J. 2022, 430, 133031. [Google Scholar] [CrossRef]
- Pawar, A.U.; Kim, C.W.; Nguyen-Le, M.-T.; Kang, Y.S. General Review on the Components and Parameters of Photoelectrochemical System for CO2 Reduction with in Situ Analysis. ACS Sustain. Chem. Eng. 2019, 7, 7431–7455. [Google Scholar] [CrossRef]
- Long, C.; Li, X.; Guo, J.; Shi, Y.; Liu, S.; Tang, Z. Electrochemical Reduction of CO2 over Heterogeneous Catalysts in Aqueous Solution: Recent Progress and Perspectives. Small Methods 2019, 3, 1800369. [Google Scholar] [CrossRef]
- Rudnev, A.V.; Zhumaev, U.E.; Kuzume, A.; Vesztergom, S.; Furrer, J.; Broekmann, P.; Wandlowski, T. The promoting effect of water on the electroreduction of CO2 in acetonitrile. Electrochim. Acta 2016, 189, 38–44. [Google Scholar] [CrossRef]
- Feng, J.; Zeng, S.; Feng, J.; Dong, H.; Zhang, X. CO2 Electroreduction in Ionic Liquids: A Review. Chin. J. Chem. 2018, 36, 961–970. [Google Scholar] [CrossRef]
- Blanchard, L.A.; Hancu, D.; Beckman, E.J.; Brennecke, J.F. Green processing using ionic liquids and CO2. Nature 1999, 399, 28–29. [Google Scholar] [CrossRef]
- Costentin, C.; Robert, M.; Savéant, J.-M. Catalysis of the electrochemical reduction of carbon dioxide. Chem. Soc. Rev. 2013, 42, 2423–2436. [Google Scholar] [CrossRef]
- Sánchez, O.G.; Birdja, Y.Y.; Bulut, M.; Vaes, J.; Breugelmans, T.; Pant, D. Recent advances in industrial CO2 electroreduction. Curr. Opin. Green Sustain. Chem. 2019, 16, 47–56. [Google Scholar] [CrossRef]
- Fan, L.; Xia, C.; Yang, F.; Wang, J.; Wang, H.; Lu, Y. Strategies in catalysts and electrolyzer design for electrochemical CO2 reduction toward C2+ products. Sci. Adv. 2020, 6, eaay3111. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S.; Winè, G.; Gangeri, M. Electrocatalytic conversion of CO2 to long carbon-chain hydrocarbons. Green Chem. 2007, 9, 671. [Google Scholar] [CrossRef]
- Pander, J.E.; Ren, D.; Huang, Y.; Loo, N.W.X.; Hong, S.H.L.; Yeo, B.S. Understanding the Heterogeneous Electrocatalytic Reduction of Carbon Dioxide on Oxide-Derived Catalysts. ChemElectroChem 2018, 5, 219–237. [Google Scholar] [CrossRef]
- Lu, Q.; Jiao, F. Electrochemical CO2 reduction: Electrocatalyst, reaction mechanism, and process engineering. Nano Energy 2016, 29, 439–456. [Google Scholar] [CrossRef]
- Birdja, Y.Y.; Pérez-Gallent, E.; Figueiredo, M.C.; Göttle, A.J.; Calle-Vallejo, F.; Koper, M.T.M. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat. Energy 2019, 4, 732–745. [Google Scholar] [CrossRef]
- Popović, S.; Smiljanić, M.; Jovanovič, P.; Vavra, J.; Buonsanti, R.; Hodnik, N. Stability and Degradation Mechanisms of Copper-Based Catalysts for Electrochemical CO2 Reduction. Angew. Chem. 2020, 132, 14844–14854. [Google Scholar] [CrossRef]
- Shan, W.; Liu, R.; Zhao, H.; Liu, J. Bicarbonate Rebalances the *COOH/*OCO—Dual Pathways in CO2 Electrocatalytic Reduction: In Situ Surface-Enhanced Raman Spectroscopic Evidence. J. Phys. Chem. Lett. 2022, 13, 7296–7305. [Google Scholar] [CrossRef]
- Yang, Y.; Roh, I.; Louisia, S.; Chen, C.; Jin, J.; Yu, S.; Salmeron, M.B.; Wang, C.; Yang, P. Operando Resonant Soft X-ray Scattering Studies of Chemical Environment and Interparticle Dynamics of Cu Nanocatalysts for CO2 Electroreduction. J. Am. Chem. Soc. 2022, 144, 8927–8931. [Google Scholar] [CrossRef]
- Bernal, M.; Bagger, A.; Scholten, F.; Sinev, I.; Bergmann, A.; Ahmadi, M.; Rossmeisl, J.; Cuenya, B.R. CO2 electroreduction on copper-cobalt nanoparticles: Size and composition effect. Nano Energy 2018, 53, 27–36. [Google Scholar] [CrossRef]
- Zhu, S.; Li, T.; Cai, W.-B.; Shao, M. CO2 Electrochemical Reduction as Probed through Infrared Spectroscopy. ACS Energy Lett. 2019, 4, 682–689. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, H.; Guo, X.; Luo, J.; Zakeeruddin, S.M.; Ren, D.; Grätzel, M. Selective C–C Coupling in Carbon Dioxide Electroreduction via Efficient Spillover of Intermediates as Supported by Operando Raman Spectroscopy. J. Am. Chem. Soc. 2019, 141, 18704–18714. [Google Scholar] [CrossRef]
- Kortlever, R.; Shen, J.; Schouten, K.J.P.; Calle-Vallejo, F.; Koper, M.T.M. Catalysts and Reaction Pathways for the Electrochemical Reduction of Carbon Dioxide. J. Phys. Chem. Lett. 2015, 6, 4073–4082. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, Q.; Li, G.; Yang, H.; He, C. Recent Advances and Perspectives of Electrochemical CO2 Reduction Toward C2+ Products on Cu-Based Catalysts. Electrochem. Energy Rev. 2022, 5, 28. [Google Scholar] [CrossRef]
- Quan, W.; Lin, Y.; Luo, Y.; Huang, Y. Electrochemical CO2 Reduction on Cu: Synthesis-Controlled Structure Preference and Selectivity. Adv. Sci. 2021, 8, 2101597. [Google Scholar] [CrossRef]
- Dattila, F.; García-Muelas, R.; López, N. Active and Selective Ensembles in Oxide-Derived Copper Catalysts for CO2 Reduction. ACS Energy Lett. 2020, 5, 3176–3184. [Google Scholar] [CrossRef]
- Kim, D.; Kley, C.S.; Li, Y.; Yang, P. Copper nanoparticle ensembles for selective electroreduction of CO2 to C2–C3 products. Proc. Natl. Acad. Sci. USA 2017, 114, 10560–10565. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y. Electrochemical CO2 Reduction on Metal Electrodes. In Modern Aspects of Electrochemistry; Vayenas, C.G., White, R.E., Gamboa-Aldeco, M.E., Eds.; Springer: New York, NY, USA, 2008; pp. 89–189. [Google Scholar] [CrossRef]
- Kumar, B.; Llorente, M.; Froehlich, J.; Dang, T.; Sathrum, A.; Kubiak, C.P. Photochemical and Photoelectrochemical Reduction of CO2. Annu. Rev. Phys. Chem. 2012, 63, 541–569. [Google Scholar] [CrossRef] [PubMed]
- Genovese, C.; Ampelli, C.; Perathoner, S.; Centi, G. Mechanism of C–C bond formation in the electrocatalytic reduction of CO2 to acetic acid. A challenging reaction to use renewable energy with chemistry. Green Chem. 2017, 19, 2406–2415. [Google Scholar] [CrossRef]
- Ramírez-Valencia, L.D.; Bailón-García, E.; Carrasco-Marín, F.; Pérez-Cadenas, A.F. From CO2 to Value-Added Products: A Review about Carbon-Based Materials for Electro-Chemical CO2 Conversion. Catalysts 2021, 11, 351. [Google Scholar] [CrossRef]
- Zhong, H.; Fujii, K.; Nakano, Y.; Jin, F. Effect of CO2 Bubbling into Aqueous Solutions Used for Electrochemical Reduction of CO2 for Energy Conversion and Storage. J. Phys. Chem. C 2015, 119, 55–61. [Google Scholar] [CrossRef]
- Marcandalli, G.; Villalba, M.; Koper, M.T.M. The Importance of Acid–Base Equilibria in Bicarbonate Electrolytes for CO2 Electrochemical Reduction and CO Reoxidation Studied on Au(hkl) Electrodes. Langmuir 2021, 37, 5707–5716. [Google Scholar] [CrossRef]
- Pérez-Gallent, E.; Marcandalli, G.; Figueiredo, M.C.; Calle-Vallejo, F.; Koper, M.T.M. Structure- and Potential-Dependent Cation Effects on CO Reduction at Copper Single-Crystal Electrodes. J. Am. Chem. Soc. 2017, 139, 16412–16419. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.C.O.; Dattila, F.; Hagedoorn, B.; García-Muelas, R.; López, N.; Koper, M.T.M. Absence of CO2 electroreduction on copper, gold and silver electrodes without metal cations in solution. Nat. Catal. 2021, 4, 654–662. [Google Scholar] [CrossRef]
- Liu, X.; Monteiro, M.C.O.; Koper, M.T.M. Interfacial pH measurements during CO2 reduction on gold using a rotating ring-disk electrode. Phys. Chem. Chem. Phys. 2023, 25, 2897–2906. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Junqueira, J.R.C.; Sikdar, N.; Öhl, D.; Dieckhöfer, S.; Quast, T.; Seisel, S.; Masa, J.; Andronescu, C.; Schuhmann, W. B-Cu-Zn Gas Diffusion Electrodes for CO2 Electroreduction to C2+ Products at High Current Densities. Angew. Chem. Int. Ed. 2021, 60, 9135–9141. [Google Scholar] [CrossRef]
- Sikdar, N.; Junqueira, J.R.C.; Dieckhöfer, S.; Quast, T.; Braun, M.; Song, Y.; Aiyappa, H.B.; Seisel, S.; Weidner, J.; Öhl, D.; et al. A Metal–Organic Framework derived CuxOYCz Catalyst for Electrochemical CO2 Reduction and Impact of Local pH Change. Angew. Chem. Int. Ed. 2021, 60, 23427–23434. [Google Scholar] [CrossRef]
- Monteiro, M.C.O.; Dieckhöfer, S.; Bobrowski, T.; Quast, T.; Pavesi, D.; Koper, M.T.M.; Schuhmann, W. Probing the local activity of CO2 reduction on gold gas diffusion electrodes: Effect of the catalyst loading and CO2 pressure. Chem. Sci. 2021, 12, 15682–15690. [Google Scholar] [CrossRef]
- Francke, R.; Schille, B.; Roemelt, M. Homogeneously Catalyzed Electroreduction of Carbon Dioxide—Methods, Mechanisms, and Catalysts. Chem. Rev. 2018, 118, 4631–4701. [Google Scholar] [CrossRef]
- Costentin, C.; Drouet, S.; Robert, M.; Savéant, J.-M. A Local Proton Source Enhances CO2 Electroreduction to CO by a Molecular Fe Catalyst. Science 2012, 338, 90–94. [Google Scholar] [CrossRef]
- Schilter, D. Homogeneous catalysis: Synthetic models close in on enzymes. Nat. Rev. Chem. 2018, 2, 147. [Google Scholar] [CrossRef]
- Costentin, C.; Passard, G.; Robert, M.; Savéant, J.-M. Ultraefficient homogeneous catalyst for the CO2-to-CO electrochemical conversion. Proc. Natl. Acad. Sci. USA 2014, 111, 14990–14994. [Google Scholar] [CrossRef]
- Ahmed, M.E.; Rana, A.; Saha, R.; Dey, S.; Dey, A. Homogeneous Electrochemical Reduction of CO2 to CO by a Cobalt Pyridine Thiolate Complex. Inorg. Chem. 2020, 59, 5292–5302. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, S.-X.; Gandionco, K.A.; Bond, A.M.; Zhang, J. Electrocatalytic carbon dioxide reduction: From fundamental principles to catalyst design. Mater. Today Adv. 2020, 7, 100074. [Google Scholar] [CrossRef]
- Pei, Y.; Zhong, H.; Jin, F. A brief review of electrocatalytic reduction of CO2—Materials, reaction conditions, and devices. Energy Sci. Eng. 2021, 9, 1012–1032. [Google Scholar] [CrossRef]
- Perry, S.C.; Leung, P.; Wang, L.; Ponce de León, C. Developments on carbon dioxide reduction: Their promise, achievements, and challenges. Curr. Opin. Electrochem. 2020, 20, 88–98. [Google Scholar] [CrossRef]
- Boettcher, S.W.; Oener, S.Z.; Lonergan, M.C.; Surendranath, Y.; Ardo, S.; Brozek, C.; Kempler, P.A. Potentially Confusing: Potentials in Electrochemistry. ACS Energy Lett. 2021, 6, 261–266. [Google Scholar] [CrossRef]
- Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012, 5, 7050–7059. [Google Scholar] [CrossRef]
- Hori, Y. CO2-reduction, catalyzed by metal electrodes. Handb. Fuel Cells 2010, 1–14. [Google Scholar] [CrossRef]
- Jhong, H.-R.M.; Ma, S.; Kenis, P.J. Electrochemical conversion of CO2 to useful chemicals: Current status, remaining challenges, and future opportunities. Curr. Opin. Chem. Eng. 2013, 2, 191–199. [Google Scholar] [CrossRef]
- Costentin, C.; Passard, G.; Savéant, J.-M. Benchmarking of Homogeneous Electrocatalysts: Overpotential, Turnover Frequency, Limiting Turnover Number. J. Am. Chem. Soc. 2015, 137, 5461–5467. [Google Scholar] [CrossRef]
- Teeter, T.E.; Van Rysselberghe, P. Reduction of Carbon Dioxide on Mercury Cathodes. J. Chem. Phys. 1954, 22, 759–760. [Google Scholar] [CrossRef]
- Hori, Y.; Kikuchi, K.; Suzuki, S. Production of CO and CH4 in Electrochemical Reduction of CO2 at Metal Electrodes in Aqueous Hydrogencarbonate Solution. Chem. Lett. 1985, 14, 1695–1698. [Google Scholar] [CrossRef]
- Hori, Y.; Murata, A.; Takahashi, R. Formation of hydrocarbons in the electrochemical reduction of carbon dioxide at a copper electrode in aqueous solution. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1989, 85, 2309. [Google Scholar] [CrossRef]
- Hori, Y.; Murata, A. Electrochemical evidence of intermediate formation of adsorbed CO in cathodic reduction of CO2 at a nickel electrode. Electrochim. Acta 1990, 35, 1777–1780. [Google Scholar] [CrossRef]
- Hori, Y.; Takahashi, R.; Yoshinami, Y.; Murata, A. Electrochemical Reduction of CO at a Copper Electrode. J. Phys. Chem. B 1997, 101, 7075–7081. [Google Scholar] [CrossRef]
- Hori, Y.; Wakebe, H.; Tsukamoto, T.; Koga, O. Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochim. Acta 1994, 39, 1833–1839. [Google Scholar] [CrossRef]
- Kostecki, R.; Augustynski, J. Electrochemical reduction of CO2 at an activated silver electrode. Berichte der Bunsengesellschaft für Phys. Chem. 1994, 98, 1510–1515. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, J.; Li, M.; Züttel, A. Boosting CO Production in Electrocatalytic CO2 Reduction on Highly Porous Zn Catalysts. ACS Catal. 2019, 9, 3783–3791. [Google Scholar] [CrossRef]
- Wu, L.; Wu, L.; Guo, C.; Guan, Y.; Wang, H.; Lu, J. Progress in Electroreduction of CO2 to Form Various Fuels Based on Zn Catalysts. Processes 2023, 11, 1039. [Google Scholar] [CrossRef]
- An, X.; Li, S.; Hao, X.; Xie, Z.; Du, X.; Wang, Z.; Hao, X.; Abudula, A.; Guan, G. Common strategies for improving the performances of tin and bismuth-based catalysts in the electrocatalytic reduction of CO2 to formic acid/formate. Renew. Sustain. Energy Rev. 2021, 143, 110952. [Google Scholar] [CrossRef]
- Xue, Y.; Guo, Y.; Cui, H.; Zhou, Z. Catalyst Design for Electrochemical Reduction of CO2 to Multicarbon Products. Small Methods 2021, 5, 2100736. [Google Scholar] [CrossRef]
- Hoshi, N.; Kato, M.; Hori, Y. Electrochemical reduction of CO2 on single crystal electrodes of silver Ag(111), Ag(100) and Ag(110). J. Electroanal. Chem. 1997, 440, 283–286. [Google Scholar] [CrossRef]
- Taguchi, S.; Aramata, A. Surface-structure sensitive reduced CO2 formation on Pt single crystal electrodes in sulfuric acid solution. Electrochim. Acta 1994, 39, 2533–2537. [Google Scholar] [CrossRef]
- Qu, Y.; Duan, X. Progress, challenge and perspective of heterogeneous photocatalysts. Chem. Soc. Rev. 2013, 42, 2568–2580. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Cheng, T.; Goddard, W.A.; Sundararaman, R. Mechanistic Explanation of the pH Dependence and Onset Potentials for Hydrocarbon Products from Electrochemical Reduction of CO on Cu (111). J. Am. Chem. Soc. 2016, 138, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Frese, K.W. Electrochemical Reduction of CO2 at Intentionally Oxidized Copper Electrodes. J. Electrochem. Soc. 1991, 138, 3338–3344. [Google Scholar] [CrossRef]
- Le, M.; Ren, M.; Zhang, Z.; Sprunger, P.T.; Kurtz, R.L.; Flake, J.C. Electrochemical Reduction of CO2 to CH3OH at Copper Oxide Surfaces. J. Electrochem. Soc. 2011, 158, E45–E49. [Google Scholar] [CrossRef]
- Gao, S.; Jiao, X.; Sun, Z.; Zhang, W.; Sun, Y.; Wang, C.; Hu, Q.; Zu, X.; Yang, F.; Yang, S.; et al. Ultrathin Co3O4 Layers Realizing Optimized CO2 Electroreduction to Formate. Angew. Chem. Int. Ed. 2016, 55, 698–702. [Google Scholar] [CrossRef]
- Giri, S.D.; Sarkar, A.; Mahajani, S.; Suresh, A.K. Electrochemical Reduction of CO2 on Copper Oxidized by Electrochemical Methods. ECS Trans. 2017, 75, 19–31. [Google Scholar] [CrossRef]
- Deng, P.; Wang, H.; Qi, R.; Zhu, J.; Chen, S.; Yang, F.; Zhou, L.; Qi, K.; Liu, H.; Xia, B.Y. Bismuth Oxides with Enhanced Bismuth–Oxygen Structure for Efficient Electrochemical Reduction of Carbon Dioxide to Formate. ACS Catal. 2020, 10, 743–750. [Google Scholar] [CrossRef]
- Gao, D.; Zhang, Y.; Zhou, Z.; Cai, F.; Zhao, X.; Huang, W.; Li, Y.; Zhu, J.; Liu, P.; Yang, F.; et al. Enhancing CO2 Electroreduction with the Metal–Oxide Interface. J. Am. Chem. Soc. 2017, 139, 5652–5655. [Google Scholar] [CrossRef]
- Dutta, A.; Kuzume, A.; Rahaman, M.; Vesztergom, S.; Broekmann, P. Monitoring the Chemical State of Catalysts for CO2 Electroreduction: An In Operando Study. ACS Catal. 2015, 5, 7498–7502. [Google Scholar] [CrossRef]
- Timoshenko, J.; Roldan Cuenya, B. In Situ/Operando Electrocatalyst Characterization by X-ray Absorption Spectroscopy. Chem. Rev. 2021, 121, 882–961. [Google Scholar] [CrossRef]
- Mistry, H.; Varela, A.S.; Bonifacio, C.S.; Zegkinoglou, I.; Sinev, I.; Choi, Y.-W.; Kisslinger, K.; Stach, E.A.; Yang, J.C.; Strasser, P.; et al. Highly selective plasma-activated copper catalysts for carbon dioxide reduction to ethylene. Nat. Commun. 2016, 7, 12123. [Google Scholar] [CrossRef]
- Eilert, A.; Cavalca, F.; Roberts, F.S.; Osterwalder, J.; Liu, C.; Favaro, M.; Crumlin, E.J.; Ogasawara, H.; Friebel, D.; Pettersson, L.G.M.; et al. Subsurface Oxygen in Oxide-Derived Copper Electrocatalysts for Carbon Dioxide Reduction. J. Phys. Chem. Lett. 2017, 8, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Wong, N.T.; Handoko, A.D.; Huang, Y.; Yeo, B.S. Mechanistic Insights into the Enhanced Activity and Stability of Agglomerated Cu Nanocrystals for the Electrochemical Reduction of Carbon Dioxide to n-Propanol. J. Phys. Chem. Lett. 2016, 7, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Lum, Y.; Clark, E.L.; Ager, J.W.; Bell, A.T. CO2 Electroreduction with Enhanced Ethylene and Ethanol Selectivity by Nanostructuring Polycrystalline Copper. ChemElectroChem 2016, 3, 1012–1019. [Google Scholar] [CrossRef]
- Ait Ahsaine, H.; Zbair, M.; BaQais, A.; Arab, M. CO2 Electroreduction over Metallic Oxide, Carbon-Based, and Molecular Catalysts: A Mini-Review of the Current Advances. Catalysts 2022, 12, 450. [Google Scholar] [CrossRef]
- Lu, Q.; Rosen, J.; Jiao, F. Nanostructured Metallic Electrocatalysts for Carbon Dioxide Reduction. ChemCatChem 2015, 7, 38–47. [Google Scholar] [CrossRef]
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Back, S.; Yeom, M.S.; Jung, Y. Active Sites of Au and Ag Nanoparticle Catalysts for CO2 Electroreduction to CO. ACS Catal. 2015, 5, 5089–5096. [Google Scholar] [CrossRef]
- Gao, D.; Zhou, H.; Wang, J.; Miao, S.; Yang, F.; Wang, G.; Wang, J.; Bao, X. Size-Dependent Electrocatalytic Reduction of CO2 over Pd Nanoparticles. J. Am. Chem. Soc. 2015, 137, 4288–4291. [Google Scholar] [CrossRef] [PubMed]
- Salehi-Khojin, A.; Jhong, H.-R.M.; Rosen, B.A.; Zhu, W.; Ma, S.; Kenis, P.J.A.; Masel, R.I. Nanoparticle Silver Catalysts That Show Enhanced Activity for Carbon Dioxide Electrolysis. J. Phys. Chem. C 2013, 117, 1627–1632. [Google Scholar] [CrossRef]
- de Lucas-Consuegra, A.; Serrano-Ruiz, J.; Gutiérrez-Guerra, N.; Valverde, J. Low-Temperature Electrocatalytic Conversion of CO2 to Liquid Fuels: Effect of the Cu Particle Size. Catalysts 2018, 8, 340. [Google Scholar] [CrossRef]
- Lates, V.; Falch, A.; Jordaan, A.; Peach, R.; Kriek, R.J. An electrochemical study of carbon dioxide electroreduction on gold-based nanoparticle catalysts. Electrochim. Acta 2014, 128, 75–84. [Google Scholar] [CrossRef]
- Herzog, A.; Bergmann, A.; Jeon, H.S.; Timoshenko, J.; Kühl, S.; Rettenmaier, C.; Lopez Luna, M.; Haase, F.T.; Roldan Cuenya, B. Operando Investigation of Ag-Decorated Cu2O Nanocube Catalysts with Enhanced CO2 Electroreduction toward Liquid Products. Angew. Chem. Int. Ed. 2021, 60, 7426–7435. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; An, X.; Park, K.H.; Khraisheh, M.; Tang, J. A critical review of CO2 photoconversion: Catalysts and reactors. Catal. Today 2014, 224, 3–12. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Y.-J.; Zhang, H.; Lv, H.; Li, Q.; Michalsky, R.; Peterson, A.A.; Sun, S. Active and Selective Conversion of CO2 to CO on Ultrathin Au Nanowires. J. Am. Chem. Soc. 2014, 136, 16132–16135. [Google Scholar] [CrossRef]
- Liu, S.; Tao, H.; Zeng, L.; Liu, Q.; Xu, Z.; Liu, Q.; Luo, J.-L. Shape-Dependent Electrocatalytic Reduction of CO2 to CO on Triangular Silver Nanoplates. J. Am. Chem. Soc. 2017, 139, 2160–2163. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, L.; Yang, P.; Hu, C.; Luo, Z.; Chang, X.; Zhao, Z.; Gong, J. Low-Coordinated Edge Sites on Ultrathin Palladium Nanosheets Boost Carbon Dioxide Electroreduction Performance. Angew. Chem. Int. Ed. 2018, 57, 11544–11548. [Google Scholar] [CrossRef]
- Li, F.; Chen, L.; Knowles, G.P.; MacFarlane, D.R.; Zhang, J. Hierarchical Mesoporous SnO2 Nanosheets on Carbon Cloth: A Robust and Flexible Electrocatalyst for CO2 Reduction with High Efficiency and Selectivity. Angew. Chem. Int. Ed. 2017, 56, 505–509. [Google Scholar] [CrossRef]
- Jerome, M.P.; Alahmad, F.A.; Salem, M.T.; Tahir, M. Layered double hydroxide (LDH) nanomaterials with engineering aspects for photocatalytic CO2 conversion to energy efficient fuels: Fundamentals, recent advances, and challenges. J. Environ. Chem. Eng. 2022, 10, 108151. [Google Scholar] [CrossRef]
- Luo, W.; Nie, X.; Janik, M.J.; Asthagiri, A. Facet Dependence of CO2 Reduction Paths on Cu Electrodes. ACS Catal. 2016, 6, 219–229. [Google Scholar] [CrossRef]
- Loiudice, A.; Lobaccaro, P.; Kamali, E.A.; Thao, T.; Huang, B.H.; Ager, J.W.; Buonsanti, R. Tailoring Copper Nanocrystals towards C2 Products in Electrochemical CO2 Reduction. Angew. Chem. Int. Ed. 2016, 55, 5789–5792. [Google Scholar] [CrossRef]
- Tang, W.; Peterson, A.A.; Varela, A.S.; Jovanov, Z.P.; Bech, L.; Durand, W.J.; Dahl, S.; Nørskov, J.K.; Chorkendorff, I. The importance of surface morphology in controlling the selectivity of polycrystalline copper for CO2 electroreduction. Phys. Chem. Chem. Phys. 2012, 14, 76–81. [Google Scholar] [CrossRef]
- Suen, N.-T.; Kong, Z.-R.; Hsu, C.-S.; Chen, H.-C.; Tung, C.-W.; Lu, Y.-R.; Dong, C.-L.; Shen, C.-C.; Chung, J.-C.; Chen, H.M. Morphology Manipulation of Copper Nanocrystals and Product Selectivity in the Electrocatalytic Reduction of Carbon Dioxide. ACS Catal. 2019, 9, 5217–5222. [Google Scholar] [CrossRef]
- Reske, R.; Mistry, H.; Behafarid, F.; Roldan Cuenya, B.; Strasser, P. Particle Size Effects in the Catalytic Electroreduction of CO2 on Cu Nanoparticles. J. Am. Chem. Soc. 2014, 136, 6978–6986. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Deng, D.; Pan, X.; Fu, Q.; Bao, X. Understanding nano effects in catalysis. Natl. Sci. Rev. 2015, 2, 183–201. [Google Scholar] [CrossRef]
- Zhu, W.; Michalsky, R.; Metin, Ö.; Lv, H.; Guo, S.; Wright, C.J.; Sun, X.; Peterson, A.A.; Sun, S. Monodisperse Au Nanoparticles for Selective Electrocatalytic Reduction of CO2 to CO. J. Am. Chem. Soc. 2013, 135, 16833–16836. [Google Scholar] [CrossRef]
- Zheng, X.; De Luna, P.; García de Arquer, F.P.; Zhang, B.; Becknell, N.; Ross, M.B.; Li, Y.; Banis, M.N.; Li, Y.; Liu, M.; et al. Sulfur-Modulated Tin Sites Enable Highly Selective Electrochemical Reduction of CO2 to Formate. Joule 2017, 1, 794–805. [Google Scholar] [CrossRef]
- Wu, Z.-Z.; Gao, F.-Y.; Gao, M.-R. Regulating the oxidation state of nanomaterials for electrocatalytic CO2 reduction. Energy Environ. Sci. 2021, 14, 1121–1139. [Google Scholar] [CrossRef]
- Xing, Z.; Hu, L.; Ripatti, D.S.; Hu, X.; Feng, X. Enhancing carbon dioxide gas-diffusion electrolysis by creating a hydrophobic catalyst microenvironment. Nat. Commun. 2021, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Yan, S.; Zhang, C.; Hu, Q.; Cheng, Z. Electroreduction of CO2 toward High Current Density. Processes 2022, 10, 826. [Google Scholar] [CrossRef]
- Nguyen, D.L.T.; Lee, C.W.; Na, J.; Kim, M.-C.; Tu, N.D.K.; Lee, S.Y.; Sa, Y.J.; Won, D.H.; Oh, H.-S.; Kim, H.; et al. Mass Transport Control by Surface Graphene Oxide for Selective CO Production from Electrochemical CO2 Reduction. ACS Catal. 2020, 10, 3222–3231. [Google Scholar] [CrossRef]
- Weng, L.-C.; Bell, A.T.; Weber, A.Z. Modeling gas-diffusion electrodes for CO2 reduction. Phys. Chem. Chem. Phys. 2018, 20, 16973–16984. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, J.; Lv, W.; Fang, H.; Wang, W. Electrochemical reduction of CO2 to formate catalyzed by electroplated tin coating on copper foam. Appl. Surf. Sci. 2016, 362, 394–398. [Google Scholar] [CrossRef]
- Kottakkat, T.; Klingan, K.; Jiang, S.; Jovanov, Z.P.; Davies, V.H.; El-Nagar, G.A.M.; Dau, H.; Roth, C. Electrodeposited AgCu Foam Catalysts for Enhanced Reduction of CO2 to CO. ACS Appl. Mater. Interfaces 2019, 11, 14734–14744. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Kas, R.; Smith, W.A.; Burdyny, T. Role of the Carbon-Based Gas Diffusion Layer on Flooding in a Gas Diffusion Electrode Cell for Electrochemical CO2 Reduction. ACS Energy Lett. 2021, 6, 33–40. [Google Scholar] [CrossRef]
- Schwartz, N.; Harrington, J.; Ziegler, K.J.; Cox, P. Effects of Electrode Support Structure on Electrode Microstructure, Transport Properties, and Gas Diffusion within the Gas Diffusion Layer. ACS Omega 2022, 7, 29832–29839. [Google Scholar] [CrossRef]
- Rabiee, H.; Ge, L.; Zhang, X.; Hu, S.; Li, M.; Yuan, Z. Gas diffusion electrodes (GDEs) for electrochemical reduction of carbon dioxide, carbon monoxide, and dinitrogen to value-added products: A review. Energy Environ. Sci. 2021, 14, 1959–2008. [Google Scholar] [CrossRef]
- Jouny, M.; Luc, W.; Jiao, F. General Techno-Economic Analysis of CO2 Electrolysis Systems. Ind. Eng. Chem. Res. 2018, 57, 2165–2177. [Google Scholar] [CrossRef]
- Wu, B.; Chen, J.; Qian, L. Recent Advances in Heterogeneous Electroreduction of CO2 on Copper-Based Catalysts. Catalysts 2022, 12, 860. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.-Z.; Tao, H.; Li, T.; Liu, Q.; Xu, Z.; Fu, X.-Z.; Luo, J.-L. Ultrathin 5-fold twinned sub-25 nm silver nanowires enable highly selective electroreduction of CO2 to CO. Nano Energy 2018, 45, 456–462. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, S.; Jiang, M.; Hu, Y.; Hu, C.; Zhang, X.; Jin, Z. Nanocapillarity and Nanoconfinement Effects of Pipet-like Bismuth@Carbon Nanotubes for Highly Efficient Electrocatalytic CO2 Reduction. Nano Lett. 2021, 21, 2650–2657. [Google Scholar] [CrossRef]
- Dutta, A.; Zelocualtecatl Montiel, I.; Kiran, K.; Rieder, A.; Grozovski, V.; Gut, L.; Broekmann, P. A Tandem (Bi2O3 → Bi met) Catalyst for Highly Efficient ec-CO2 Conversion into Formate: Operando Raman Spectroscopic Evidence for a Reaction Pathway Change. ACS Catal. 2021, 11, 4988–5003. [Google Scholar] [CrossRef]
- Feng, X.; Zou, H.; Zheng, R.; Wei, W.; Wang, R.; Zou, W.; Lim, G.; Hong, J.; Duan, L.; Chen, H. Bi2O3/BiO2 Nanoheterojunction for Highly Efficient Electrocatalytic CO2 Reduction to Formate. Nano Lett. 2022, 22, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhou, Y.; Liu, D.; Qi, R.; Xia, C.; Li, M.; You, B.; Xia, B.Y. Carbon-Confined Indium Oxides for Efficient Carbon Dioxide Reduction in a Solid-State Electrolyte Flow Cell. Angew. Chem. Int. Ed. 2022, 61, e202200552. [Google Scholar] [CrossRef]
- Li, G.; Song, Y.; Zhu, C.; Dong, X.; Chen, W.; Wu, G.; Feng, G.; Li, S.; Wei, W. Facet-oriented Cu2O and oxygen vacancies synergistically promoting CO2 electroreduction to formate on Cu-based hollow fiber. J. CO2 Util. 2023, 70, 102446. [Google Scholar] [CrossRef]
- Han, L.; Song, S.; Liu, M.; Yao, S.; Liang, Z.; Cheng, H.; Ren, Z.; Liu, W.; Lin, R.; Qi, G.; et al. Stable and Efficient Single-Atom Zn Catalyst for CO2 Reduction to CH4. J. Am. Chem. Soc. 2020, 142, 12563–12567. [Google Scholar] [CrossRef]
- Yang, D.; Zhu, Q.; Chen, C.; Liu, H.; Liu, Z.; Zhao, Z.; Zhang, X.; Liu, S.; Han, B. Selective electroreduction of carbon dioxide to methanol on copper selenide nanocatalysts. Nat. Commun. 2019, 10, 677. [Google Scholar] [CrossRef]
- Xu, H.; Rebollar, D.; He, H.; Chong, L.; Liu, Y.; Liu, C.; Sun, C.-J.; Li, T.; Muntean, J.V.; Winans, R.E.; et al. Highly selective electrocatalytic CO2 reduction to ethanol by metallic clusters dynamically formed from atomically dispersed copper. Nat. Energy 2020, 5, 623–632. [Google Scholar] [CrossRef]
- Peng, C.; Luo, G.; Zhang, J.; Chen, M.; Wang, Z.; Sham, T.-K.; Zhang, L.; Li, Y.; Zheng, G. Double sulfur vacancies by lithium tuning enhance CO2 electroreduction to n-propanol. Nat. Commun. 2021, 12, 1580. [Google Scholar] [CrossRef] [PubMed]
- Marepally, B.C.; Ampelli, C.; Genovese, C.; Tavella, F.; Veyre, L.; Quadrelli, E.A.; Perathoner, S.; Centi, G. Role of small Cu nanoparticles in the behaviour of nanocarbon-based electrodes for the electrocatalytic reduction of CO2. J. CO2 Util. 2017, 21, 534–542. [Google Scholar] [CrossRef]
- Gualandi, I.; Vlamidis, Y.; Mazzei, L.; Musella, E.; Giorgetti, M.; Christian, M.; Morandi, V.; Scavetta, E.; Tonelli, D. Ni/Al Layered Double Hydroxide and Carbon Nanomaterial Composites for Glucose Sensing. ACS Appl. Nano Mater. 2019, 2, 143–155. [Google Scholar] [CrossRef]
- Tonelli, D.; Gualandi, I.; Musella, E.; Scavetta, E. Synthesis and Characterization of Layered Double Hydroxides as Materials for Electrocatalytic Applications. Nanomaterials 2021, 11, 725. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Mariani, F.; Fasolini, A.; Brandi, E.T.; Scavetta, E.; Basile, F.; Tonelli, D. Electrosynthesized CuMgAl Layered Double Hydroxides as New Catalysts for the Electrochemical Reduction of CO2. Adv. Funct. Mater. 2023, 2300345. [Google Scholar] [CrossRef]
- Clark, E.L.; Resasco, J.; Landers, A.; Lin, J.; Chung, L.-T.; Walton, A.; Hahn, C.; Jaramillo, T.F.; Bell, A.T. Standards and Protocols for Data Acquisition and Reporting for Studies of the Electrochemical Reduction of Carbon Dioxide. ACS Catal. 2018, 8, 6560–6570. [Google Scholar] [CrossRef]







| Drivers of Climate Change | Radiative Forcing Estimates (W m−2) |
|---|---|
| CO2 | 1.7 ± 0.4 |
| CH4 | 1.0 ± 0.2 |
| N2O | 0.2 ± 0.1 |
| Halocarbons (O3, CFCs, HCFCs) | 0.2 ± 0.2 |
| CO | 0.2 ± 0.1 |
| Solar irradiance | 0.1 ± 0.1 |
| CO2 Conversion | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Thermochemical |
|
| [21,28,29] |
| Biochemical |
|
| [23,30,31] |
| Photochemical |
|
| [32] |
| Electrochemical |
|
| [33,34,35] |
| Product | Benchmark Catalyst | Jtot (mA cm−2) | FE (%) | Ref. |
|---|---|---|---|---|
| CO | 5-fold twinned Ag NWs | ~2.2 | 99.3 | [151] |
| HCOOH | Bi-NRs@NCNTs | 6.0 | ~91 | [152] |
| CH4 | SA-Zn/MNC | 31.8 | 85 | [157] |
| CH3OH | Cu1.63Se(1/3) | 41.4 | 77.6 | [158] |
| CH3CH2OH | Cun (n = 3 and 4) cluster | ~2.0 | 91 | [159] |
| n-propanol | CuSX—DSV | 9.9 | 15.4 | [160] |
| CH3COOH | CuMgAl LDH/CP | ~0.3 | 84 | [164] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serafini, M.; Mariani, F.; Basile, F.; Scavetta, E.; Tonelli, D. From Traditional to New Benchmark Catalysts for CO2 Electroreduction. Nanomaterials 2023, 13, 1723. https://doi.org/10.3390/nano13111723
Serafini M, Mariani F, Basile F, Scavetta E, Tonelli D. From Traditional to New Benchmark Catalysts for CO2 Electroreduction. Nanomaterials. 2023; 13(11):1723. https://doi.org/10.3390/nano13111723
Chicago/Turabian StyleSerafini, Martina, Federica Mariani, Francesco Basile, Erika Scavetta, and Domenica Tonelli. 2023. "From Traditional to New Benchmark Catalysts for CO2 Electroreduction" Nanomaterials 13, no. 11: 1723. https://doi.org/10.3390/nano13111723
APA StyleSerafini, M., Mariani, F., Basile, F., Scavetta, E., & Tonelli, D. (2023). From Traditional to New Benchmark Catalysts for CO2 Electroreduction. Nanomaterials, 13(11), 1723. https://doi.org/10.3390/nano13111723