nZVI-Based Nanomaterials Used for Phosphate Removal from Aquatic Systems
Abstract
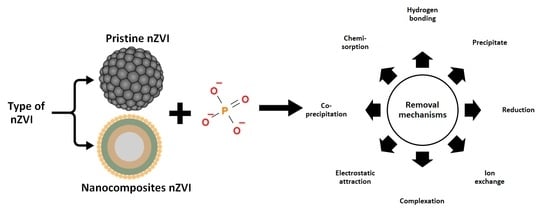
:1. Introduction
2. Synthesis of Pristine nZVI
2.1. Top-Down Methods
2.2. Bottom-Up Methods
3. Nanocomposites
3.1. nZVI Stabilized with Organic Molecules
3.2. Encapsulated nZVI
3.3. nZVI Supported/Immobilized on Organic or Inorganic Materials
3.4. Bimetallic nZVI
4. Experimental Conditions on Phosphate Adsorption Efficiency
4.1. Effect of Initial Solution pH
4.2. Effect of Oxygen
4.3. Effect of Reaction Time
4.4. Effect of Ionic Strength
4.5. Effect of Dosage of Adsorbent
4.6. Effect of Initial Concentration of Phosphate
4.7. Interferences
4.8. Regeneration
4.9. Temperature
5. Relationship between Maximum Adsorption Capacity and Partition Coefficient
6. Risks and (Dis)advantages Associated with the Use of nZVI
7. Summary
8. Conclusions and Projections
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cordell, D.; White, S. Peak Phosphorus: Clarifying the Key Issues of a Vigorous Debate about Long-Term Phosphorus Security. Sustainability 2011, 3, 2027–2049. [Google Scholar] [CrossRef] [Green Version]
- Wan, J.; Wu, B.; Lo, I.M.C. Development of Fe0/Fe3O4 Composites with Tunable Properties Facilitated by Fe2+ for Phosphate Removal from River Water. Chem. Eng. J. 2020, 388, 124242. [Google Scholar] [CrossRef]
- Gibbons, W.A.; Siemion, I.Z. Agricultural Phosphorus and Eutrophication: A Symposium Overview. Biophys. Chem. 1988, 27, 251–257. [Google Scholar] [CrossRef]
- Nisbeth, C.S.; Kidmose, J.; Weckström, K.; Reitzel, K.; Odgaard, B.V.; Bennike, O.; Thorling, L.; McGowan, S.; Schomacker, A.; Juul Kristensen, D.L.; et al. Dissolved Inorganic Geogenic Phosphorus Load to a Groundwater-Fed Lake: Implications of Terrestrial Phosphorus Cycling by Groundwater. Water 2019, 11, 2213. [Google Scholar] [CrossRef] [Green Version]
- Zak, D.; Kronvang, B.; Carstensen, M.V.; Hoffmann, C.C.; Kjeldgaard, A.; Larsen, S.E.; Audet, J.; Egemose, S.; Jorgensen, C.A.; Feuerbach, P.; et al. Nitrogen and Phosphorus Removal from Agricultural Runoff in Integrated Buffer Zones. Environ. Sci. Technol. 2018, 52, 6508–6517. [Google Scholar] [CrossRef] [Green Version]
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E.; Lancelot, C.; Likens, G.E. Ecology—Controlling Eutrophication: Nitrogen and Phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Owens, L.B.; Shipitalo, M.J. Surface and Subsurface Phosphorus Losses from Fertilized Pasture Systems in Ohio. J. Environ. Qual. 2006, 35, 1101–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, B.; Wan, J.; Zhang, Y.; Pan, B.; Lo, I.M.C. Selective Phosphate Removal from Water and Wastewater Using Sorption: Process Fundamentals and Removal Mechanisms. Environ. Sci. Technol. 2020, 54, 50–66. [Google Scholar] [CrossRef]
- Rathod, M.; Mody, K.; Basha, S. Efficient Removal of Phosphate from Aqueous Solutions by Red Seaweed, Kappaphycus Alverezii. J. Clean. Prod. 2014, 84, 484–493. [Google Scholar] [CrossRef]
- Li, X.; Xie, Y.; Jiang, F.; Wang, B.; Hu, Q.; Tang, Y.; Luo, T.; Wu, T. Enhanced Phosphate Removal from Aqueous Solution Using Resourceable Nano-CaO2/BC Composite: Behaviors and Mechanisms Precipitation. Sci. Total Environ. 2020, 709, 136123. [Google Scholar] [CrossRef]
- Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Bolan, N.S. Removal and Recovery of Phosphate from Water Using Sorption. Crit. Rev. Environ. Sci. Technol. 2014, 44, 847–907. [Google Scholar] [CrossRef]
- Rashidi Nodeh, H.; Sereshti, H.; Zamiri Afsharian, E.; Nouri, N. Enhanced Removal of Phosphate and Nitrate Ions from Aqueous Media Using Nanosized Lanthanum Hydrous Doped on Magnetic Graphene Nanocomposite. J. Environ. Manag. 2017, 197, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Bhateria, R.; Singh, R. A Review on Nanotechnological Application of Magnetic Iron Oxides for Heavy Metal Removal. J. Water Process. Eng. 2019, 31, 100845. [Google Scholar] [CrossRef]
- Mueller, N.C.; Nowack, B. Nanoparticles for Remediation: Solving Big Problems with Little Particles. Elements 2010, 6, 395–400. [Google Scholar] [CrossRef]
- Carroll, D.O.; Sleep, B.; Krol, M.; Boparai, H.; Kocur, C. Nanoscale Zero Valent Iron and Bimetallic Particles for Contaminated Site Remediation. Adv. Water Resour. 2013, 51, 104–122. [Google Scholar] [CrossRef]
- Ju-Nam, Y.; Lead, J.R. Manufactured Nanoparticles: An Overview of Their Chemistry, Interactions and Potential Environmental Implications. Sci. Total Environ. 2008, 400, 396–414. [Google Scholar] [CrossRef]
- Abraham, P.M.; Barnikol, S.; Baumann, T.; Kuehn, M.; Ivleva, N.P.; Schaumann, G.E. Sorption of Silver Nanoparticles to Environmental and Model Surfaces. Environ. Sci. Technol. 2013, 47, 5083–5091. [Google Scholar] [CrossRef]
- Liu, G.; Han, C.; Kong, M.; Abdelraheem, W.H.M.; Nadagouda, M.N.; Dionysiou, D.D. Nanoscale Zero-Valent Iron Confined in Anion Exchange Resins to Enhance Selective Adsorption of Phosphate from Wastewater. ACS EST Eng. 2022, 2, 1454–1464. [Google Scholar] [CrossRef]
- Bae, S.; Hanna, K. Reactivity of Nanoscale Zero-Valent Iron in Unbuffered Systems: Effect of pH and Fe(II) Dissolution. Environ. Sci. Technol. 2015, 49, 10536–10543. [Google Scholar] [CrossRef]
- Nagoya, S.; Nakamichi, S.; Kawase, Y. Mechanisms of Phosphate Removal from Aqueous Solution by Zero-Valent Iron: A Novel Kinetic Model for Electrostatic Adsorption, Surface Complexation and Precipitation of Phosphate under Oxic Conditions. Sep. Purif. Technol. 2019, 218, 120–129. [Google Scholar] [CrossRef]
- Wang, L.; Putnis, C.V.; Hövelmann, J.; Putnis, A. Interfacial Precipitation of Phosphate on Hematite and Goethite. Minerals 2018, 8, 207. [Google Scholar] [CrossRef] [Green Version]
- Wen, Z.; Zhang, Y.; Dai, C. Removal of Phosphate from Aqueous Solution Using Nanoscale Zerovalent Iron (nZVI). Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 433–440. [Google Scholar] [CrossRef]
- Mukherjee, R.; Kumar, R.; Sinha, A.; Lama, Y.; Saha, A.K. A Review on Synthesis, Characterization, and Applications of Nano Zero Valent Iron (nZVI) for Environmental Remediation. Crit. Rev. Environ. Sci. Technol. 2016, 46, 443–466. [Google Scholar] [CrossRef]
- Mahmoud, A.S.; Mostafa, M.K.; Nasr, M. Regression Model, Artificial Intelligence, and Cost Estimation for Phosphate Adsorption Using Encapsulated Nanoscale Zero-Valent Iron. Sep. Sci. Technol. 2019, 54, 13–26. [Google Scholar] [CrossRef]
- Zhang, X.; Navarathna, C.M.; Leng, W.; Karunaratne, T.; Thirumalai, R.V.K.G.; Kim, Y.; Pittman, C.U.; Mlsna, T.; Cai, Z.; Zhang, J. Lignin-Based Few-Layered Graphene-Encapsulated Iron Nanoparticles for Water Remediation. Chem. Eng. J. 2021, 417, 129199. [Google Scholar] [CrossRef]
- Khalil, A.M.E.; Eljamal, O.; Amen, T.W.M.; Sugihara, Y.; Matsunaga, N. Optimized Nano-Scale Zero-Valent Iron Supported on Treated Activated Carbon for Enhanced Nitrate and Phosphate Removal from Water. Chem. Eng. J. 2017, 309, 349–365. [Google Scholar] [CrossRef]
- Kozma, G.; Rónavári, A.; Kónya, Z.; Kukovecz, Á. Environmentally Benign Synthesis Methods of Zero-Valent Iron Nanoparticles. ACS Sustain. Chem. Eng. 2016, 4, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Chen, T.; Zou, X.; Xie, Q.; Qing, C.; Chen, D.; Frost, R.L. Removal of Phosphorus Using NZVI Derived from Reducing Natural Goethite. Chem. Eng. J. 2013, 234, 80–87. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Liu, H.; Chen, T.; Chen, D.; Li, M.; Chen, C. The Synthesis of NZVI and Its Application to the Removal of Phosphate from Aqueous Solutions. Water Air Soil Pollut. 2017, 228, 321. [Google Scholar] [CrossRef]
- Maamoun, I.; Eljamal, O.; Khalil, A.M.E.; Sugihara, Y.; Matsunaga, N. Phosphate Removal Through Nano-Zero-Valent Iron Permeable Reactive Barrier; Column Experiment and Reactive Solute Transport Modeling. Transp. Porous Media 2018, 125, 395–412. [Google Scholar] [CrossRef]
- Shanableh, A.; Darwish, N.; Bhattacharjee, S.; Al-Khayyat, G.; Khalil, M.; Mousa, M.; Tayara, A.; Al-Samarai, M. Phosphorous Removal by Nanoscale Zero-Valent Iron (nZVI) and Chitosan-Coated Nzvi (Cs-nZVI). Desalination Water Treat. 2020, 184, 282–291. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Darwish, N.; Shanableh, A. Phosphate Removal Using Nanoscale Zerovalent Iron: Impact of Chitosan and Humic Acid. J. Environ. Chem. Eng. 2020, 8, 104131. [Google Scholar] [CrossRef]
- Wu, D.; Shen, Y.; Ding, A.; Qiu, M.; Yang, Q.; Zheng, S. Phosphate Removal from Aqueous Solutions by Nanoscale Zero-Valent Iron. Environ. Technol. 2013, 34, 2663–2669. [Google Scholar] [CrossRef] [PubMed]
- Almeelbi, T.; Bezbaruah, A. Aqueous Phosphate Removal Using Nanoscale Zero-Valent Iron. J. Nanoparticle Res. 2012, 14, 197–210. [Google Scholar] [CrossRef]
- Eljamal, O.; Khalil, A.M.E.; Sugihara, Y.; Matsunaga, N. Phosphorus Removal from Aqueous Solution by Nanoscale Zero Valent Iron in the Presence of Copper Chloride. Chem. Eng. J. 2016, 293, 225–231. [Google Scholar] [CrossRef]
- Eljamal, R.; Eljamal, O.; Maamoun, I.; Yilmaz, G.; Sugihara, Y. Enhancing the Characteristics and Reactivity of nZVI: Polymers Effect and Mechanisms. J. Mol. Liq. 2020, 315, 113714. [Google Scholar] [CrossRef]
- Lin, D.; Hu, L.; Lo, I.M.C.; Yu, Z. Size Distribution and Phosphate Removal Capacity of Nano Zero-Valent Iron (nZVI): Influence of pH and Ionic Strength. Water 2020, 12, 2939. [Google Scholar] [CrossRef]
- Zheng, H.; Ren, X.; Zhang, X.; Song, G.; Chen, D.; Chen, C. Mutual Effect of U(VI) and Phosphate on the Reactivity of Nanoscale Zero-Valent Iron (nZVI) for Their Co-Removal. J. Mol. Liq. 2020, 297, 111853. [Google Scholar] [CrossRef]
- Maamoun, I.; Eljamal, R.; Falyouna, O.; Bensaida, K.; Sugihara, Y.; Eljamal, O. Insights into Kinetics, Isotherms and Thermodynamics of Phosphorus Sorption onto Nanoscale Zero-Valent Iron. J. Mol. Liq. 2021, 328, 115402. [Google Scholar] [CrossRef]
- Liu, F.; Yang, J.H.; Zuo, J.; Ma, D.; Gan, L.; Xie, B.; Wang, P.; Yang, B. Graphene-Supported Nanoscale Zero-Valent Iron: Removal of Phosphorus from Aqueous Solution and Mechanistic Study. J. Environ. Sci. 2014, 26, 1751–1762. [Google Scholar] [CrossRef]
- Xie, B.; Zuo, J.; Gan, L.; Liu, F.; Wang, K. Cation Exchange Resin Supported Nanoscale Zero-Valent Iron for Removal of Phosphorus in Rainwater Runoff. Front. Environ. Sci. Eng. 2014, 8, 463–470. [Google Scholar] [CrossRef]
- Almeelbi, T.; Haugstad, M.; Bezbaruah, A. Aqueous Phosphate Removal Using Bare and Ca-Alginate Entrapped Nanoscale Zero-Valent Iron. In Proceedings of the World Environmental and Water Resources Congress: Bearing Knowledge for Sustainability, Palm Springs, CA, USA, 22–26 May 2011; pp. 1733–1740. [Google Scholar]
- Elshfai, M.M.; Mahmoud, A.S.; Elsaid, M.A. Comparative Studies of Using nZVI and Entrapped nZVI in Alginate Biopolymer (Ag/nZVI) for Aqueous Phosphate Removal. In Proceedings of the 12th International Conference on Nano-Techology for Green Sustainable Construction, Sharm El-Sheikh, Egypt, 18–22 March 2021; pp. 1–21. [Google Scholar]
- Chen, D.; Gao, B.; Wang, H.; Yang, K. Effective Removal of High Concentration of Phosphate by Starch-Stabilized Nanoscale Zerovalent Iron (SNZVI). J. Taiwan Inst. Chem. Eng. 2016, 61, 181–187. [Google Scholar] [CrossRef]
- Eljamal, O.; Thompson, I.P.; Maamoun, I.; Shubair, T.; Kareman, E.; Lueangwattanapong, K.; Sugihara, Y. Investigating the Design Parameters for a Permeable Reactive Barrier Consisting of Nanoscale Zero-Valent Iron and Bimetallic Iron/Copper for Phosphate Removal. J. Mol. Liq. 2020, 299, 112144. [Google Scholar] [CrossRef]
- Gan, L.; Zuo, J.; Xie, B.; Li, P.; Huang, X. Zeolite (Na) Modified by Nano-Fe Particles Adsorbing Phosphate in Rainwater Runoff. J. Environ. Sci. 2012, 24, 1929–1933. [Google Scholar] [CrossRef] [PubMed]
- Som, I.; Suman, S.; Roy, M.; Gupta, S.; Saha, R. Effective Phosphate Removal from Sewage Water Using Zerovalent Iron Nanomaterial as an Adsorbent. Water Qual. Res. J. 2022, 57, 177–199. [Google Scholar] [CrossRef]
- Soliemanzadeh, A.; Fekri, M.; Bakhtiary, S.; Hejazi, M. Biosynthesis of Iron Nanoparticles and Their Application in Removing Phosphorus from Aqueous Solutions. Chem. Ecol. 2016, 32, 286–300. [Google Scholar] [CrossRef]
- Abida, O.; Van der Graaf, F.; Li, L.Y. Exploratory Study of Removing Nutrients from Aqueous Environments Employing a Green Synthesised Nano Zero-Valent Iron. Environ. Technol. 2020, 43, 2017–2032. [Google Scholar] [CrossRef]
- Farag, R.S.; Elshfai, M.M.; Mahmoud, A.S.; Mostafa, M.K.; Peters, R.W. Green Synthesis of Nano Iron Carbide: Preparation, Characterization and Application for Aqueous Phosphate Removal. In Proceedings of the 2018 Annual AIChE Meeting, Pittsburgh, PA, USA, 28 October–2 November 2018; pp. 307–317. [Google Scholar]
- Phenrat, T.; Saleh, N.; Sirk, K.; Tilton, R.D.; Lowry, G.V. Aggregation and Sedimentation of Aqueous Nanoscale Zerovalent Iron Dispersions. Environ. Sci. Technol. 2007, 41, 284–290. [Google Scholar] [CrossRef]
- Hoag, G.E.; Collins, J.B.; Holcomb, J.L.; Hoag, J.R.; Nadagouda, M.N.; Varma, R.S. Degradation of Bromothymol Blue by “greener” Nano-Scale Zero-Valent Iron Synthesized Using Tea Polyphenols. J. Mater. Chem. 2009, 19, 8671–8677. [Google Scholar] [CrossRef]
- Machado, S.; Stawiński, W.; Slonina, P.; Pinto, A.R.; Grosso, J.P.; Nouws, H.P.A.; Albergaria, J.T.; Delerue-Matos, C. Application of Green Zero-Valent Iron Nanoparticles to the Remediation of Soils Contaminated with Ibuprofen. Sci. Total Environ. 2013, 461, 323–329. [Google Scholar] [CrossRef]
- Hür, C.; Erken, E. Assessment of Green Tea-Enabled Iron/Calcined Bentonite Nanocomposites for Phosphate Removal and Recovery. J. Environ. Chem. Eng. 2022, 10, 108519. [Google Scholar] [CrossRef]
- Huang, L.; Luo, F.; Chen, Z.; Megharaj, M.; Naidu, R. Green Synthesized Conditions Impacting on the Reactivity of Fe NPs for the Degradation of Malachite Green. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Njagi, E.C.; Huang, H.; Stafford, L.; Genuino, H.; Galindo, H.M.; Collins, J.B.; Hoag, G.E.; Suib, S.L. Biosynthesis of Iron and Silver Nanoparticles at Room Temperature Using Aqueous Sorghum Bran Extracts. Langmuir 2011, 27, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Iron Complex Nanoparticles Synthesized by Eucalyptus Leaves. ACS Sustain. Chem. Eng. 2013, 1, 1551–1554. [Google Scholar] [CrossRef]
- Arshadi, M.; Foroughifard, S.; Etemad Gholtash, J.; Abbaspourrad, A. Preparation of Iron Nanoparticles-Loaded Spondias Purpurea Seed Waste as an Excellent Adsorbent for Removal of Phosphate from Synthetic and Natural Waters. J. Colloid Interface Sci. 2015, 452, 69–77. [Google Scholar] [CrossRef]
- Soliemanzadeh, A.; Fekri, M. Synthesis of Clay-Supported Nanoscale Zero-Valent Iron Using Green Tea Extract for the Removal of Phosphorus from Aqueous Solutions. Chin. J. Chem. Eng. 2017, 25, 924–930. [Google Scholar] [CrossRef]
- Arshadi, M.; Abdolmaleki, M.K.; Eskandarloo, H.; Azizi, M.; Abbaspourrad, A. Synthesis of Highly Monodispersed, Stable, and Spherical NZVI of 20-30 Nm on Filter Paper for the Removal of Phosphate from Wastewater: Batch and Column Study. ACS Sustain. Chem. Eng. 2018, 6, 11662–11676. [Google Scholar] [CrossRef]
- Zhou, R.; Li, H.; Yu, J.; Chi, R. Nanoscale Zero-Valent-Iron-Loaded Sugarcane Bagasse Composite as an Efficient Adsorbent for Phosphate Sorption from Aqueous Solution. Int. J. Environ. Sci. Technol. 2022, 20, 451–460. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, K.P. Optimization of Phosphate Removal from Aqueous Solution Using Activated Carbon Supported Zero-Valent Iron Nanoparticles: Application of RSM Approach. Appl. Water Sci. 2018, 8, 226. [Google Scholar] [CrossRef] [Green Version]
- Ma, F.; Zhao, B.; Diao, J.; Jiang, Y.; Zhang, J. Mechanism of Phosphate Removal from Aqueous Solutions by Biochar Supported Nanoscale Zero-Valent Iron. RSC Adv. 2020, 10, 39217–39225. [Google Scholar] [CrossRef]
- Arshadi, M.; Gholtash, J.E.; Zandi, H.; Foroughifard, S. Phosphate Removal by a Nano-Biosorbent from the Synthetic and Real (Persian Gulf) Water Samples. RSC Adv. 2015, 5, 43290–43302. [Google Scholar] [CrossRef]
- Ai, D.; Wei, T.; Meng, Y.; Chen, X.; Wang, B. Ball Milling Sulfur-Doped Nano Zero-Valent Iron @biochar Composite for the Efficient Removal of Phosphorus from Water: Performance and Mechanisms. Bioresour. Technol. 2022, 357, 127316. [Google Scholar] [CrossRef] [PubMed]
- Adly, A.; Mostafa, N.G.; Elawwad, A. Adsorption of Phosphorus onto Nanoscale Zero-Valent Iron/Activated Carbon: Removal Mechanisms, Thermodynamics, and Interferences. Water Reuse 2022, 12, 111–130. [Google Scholar] [CrossRef]
- Malakootian, M.; Daneshkhah, M.; Hossaini, H. Removal of Phosphates from Aqueous Solution by Sepiolite-Nano Zero Valent Iron Composite Optimization with Response Surface Methodology. Int. J. Environ. Sci. Technol. 2018, 15, 2129–2140. [Google Scholar] [CrossRef]
- Amiri, M.J. Synthesis and Optimization of Spherical nZVI (20–60 nm) Immobilized in Bio-Apatite-Based Material for Efficient Removal of Phosphate: Box-Behnken Design in a Fixed-Bed Column. Environ. Sci. Pollut. Res. 2022, 29, 67751–67764. [Google Scholar] [CrossRef]
- Shen, Z.; Dong, X.; Shi, J.; Ma, Y.; Liu, D.; Fan, J. Simultaneous Removal of Nitrate/Phosphate with Bimetallic Nanoparticles of Fe Coupled with Copper or Nickel Supported on Chelating Resin. Environ. Sci. Pollut. Res. 2019, 26, 16568–16576. [Google Scholar] [CrossRef]
- He, Y.; Lin, H.; Dong, Y.; Li, B.; Wang, L.; Chu, S.; Liu, J.; Lin, H.; Dong, Y.; Li, B.; et al. Zeolite Supported Fe/Ni Bimetallic Nanoparticles for Simultaneous Removal of Nitrate and Phosphate: Synergistic Effect and Mechanism. Chem. Eng. J. 2018, 347, 669–681. [Google Scholar] [CrossRef]
- Banerjee, A.; Nayak, D.; Lahiri, S. A New Method of Synthesis of Iron Doped Calcium Alginate Beads and Determination of Iron Content by Radiometric Method. Biochem. Eng. J. 2007, 33, 260–262. [Google Scholar] [CrossRef]
- Tratnyek, P.; Scherer, M.; Johnson, T.; Matheson, L. Permeable Reactive Barriers of Iron and Other Zero- Valent Metals. In Chemical Degradation Methods for Wastes and Pollutants; Marcel Dekker: New York, NY, USA, 2003; Volume 37, pp. 1818–1830. [Google Scholar] [CrossRef]
- Takami, S.; Eljamal, O.; Khalil, A.M.E.; Eljamal, R.; Matsunaga, N. Development of Continuous System Based on Nanoscale Zero Valent Iron Particles for Phosphorus Removal. J. Jpn. Soc. Civ. Eng. 2019, 7, 30–42. [Google Scholar] [CrossRef]
- Marková, Z.; Šišková, K.M.H.; Filip, J.; Čuda, J.; Kolář, M.; Šafářová, K.; Medřík, I.; Zbořil, R. Air Stable Magnetic Bimetallic Fe-Ag Nanoparticles for Advanced Antimicrobial Treatment and Phosphorus Removal. Environ. Sci. Technol. 2013, 47, 5285–5293. [Google Scholar] [CrossRef]
- Sleiman, N.; Deluchat, V.; Wazne, M.; Mallet, M.; Courtin-Nomade, A.; Kazpard, V.; Baudu, M. Phosphate Removal from Aqueous Solution Using ZVI/Sand Bed Reactor: Behavior and Mechanism. Water Res. 2016, 99, 56–65. [Google Scholar] [CrossRef]
- Wan, J.; Jiang, X.; Zhang, T.C.; Hu, J.; Richter-egger, D.; Zhou, A.; Tao, T. The Activated Iron System for Phosphorus Recovery in Aqueous Environments. Chemosphere 2018, 196, 153–160. [Google Scholar] [CrossRef]
- Li, X.; Huang, L.; Fang, H.; He, G.; Reible, D.; Wang, C. Immobilization of Phosphorus in Sediments by Nano Zero-Valent Iron (nZVI) from the View of Mineral Composition. Sci. Total Environ. 2019, 694, 133695. [Google Scholar] [CrossRef]
- Sleiman, N.; Deluchat, V.; Wazne, M.; Mallet, M.; Courtin-Nomade, A.; Kazpard, V.; Baudu, M. Phosphate Removal from Aqueous Solutions Using Zero Valent Iron (ZVI): Influence of Solution Composition and ZVI Aging. Colloids Surf. A Physicochem. Eng. Asp. 2017, 514, 1–10. [Google Scholar] [CrossRef]
- Eljamal, O.; Mokete, R.; Matsunaga, N.; Sugihara, Y. Chemical Pathways of Nanoscale Zero-Valent Iron (NZVI) during Its Transformation in Aqueous Solutions. Environ. Eng. Sci. Environ. Sci. 2018, 6, 6207–6220. [Google Scholar] [CrossRef]
- Baskar, A.V.; Bolan, N.; Hoang, S.A.; Sooriyakumar, P.; Kumar, M.; Singh, L.; Jasemizad, T.; Padhye, L.P.; Singh, G.; Vinu, A.; et al. Recovery, Regeneration and Sustainable Management of Spent Adsorbents from Wastewater Treatment Streams: A Review. Sci. Total Environ. 2022, 822, 153555. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Anagnostopoulos, V.A.; Bhatnagar, A.; Mitropoulos, A.C.; Kyzas, G.Z. A Review for Chromium Removal by Carbon Nanotubes. Chem. Ecol. 2017, 33, 572–588. [Google Scholar] [CrossRef]
- Lu, J.; Liu, D.; Hao, J.; Zhang, G.; Lu, B. Phosphate Removal from Aqueous Solutions by a Nano-Structured Fe-Ti Bimetal Oxide Sorbent. Chem. Eng. Res. Des. 2015, 93, 652–661. [Google Scholar] [CrossRef]
- Szulejko, J.E.; Kim, K.; Parise, J. Seeking the Most Powerful and Practical Real-World Sorbents for Gaseous Benzene as a Representative Volatile Organic Compound Based on Performance Metric. Sep. Purif. Technol. 2019, 212, 980–985. [Google Scholar] [CrossRef]
- Deng, Y.; Ok, Y.S.; Mohan, D.; Pittman, C.U.; Dou, X. Carbamazepine Removal from Water by Carbon Dot-modified Magnetic Carbon Nanotubes. Environ. Res. 2019, 169, 434–444. [Google Scholar] [CrossRef]
- Huang, Y.; Lee, X.; Grattieri, M.; Yuan, M.; Cai, R.; Macazo, F.C.; Minteer, S.D. Modified Biochar for Phosphate Adsorption in Environmentally Relevant Conditions. Chem. Eng. J. 2020, 380, 122375. [Google Scholar] [CrossRef]
- Tosco, T.; Petrangeli, M.; Cruz, C.; Sethi, R. Nanoscale Zerovalent Iron Particles for Groundwater Remediation: A Review. J. Clean. Prod. 2014, 77, 10–21. [Google Scholar] [CrossRef]
- Westerhoff, P. Engineered Nanomaterials as Emerging Contaminants in Water. In Nanotechnologies for Water Environment Applications; ASCE Press: Reston, VA, USA, 2009; p. 558. [Google Scholar]
- Galdames, A.; Ruiz-Rubio, L.; Orueta, M.; Sánchez-Arzalluz, M.; Vilas-Vilela, J.L. Zero-Valent Iron Nanoparticles for Soil and Groundwater Remediation. Int. J. Environ. Res. Public Health 2020, 17, 5817. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.I.A.; Krol, M.M.; Kocur, C.M.; Boparai, H.K.; Weber, K.P.; Sleep, B.E.; O’Carroll, D.M. nZVI Injection into Variably Saturated Soils: Field and Modeling Study. J. Contam. Hydrol. 2015, 183, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Radziemska, M.; Gusiatin, Z.M.; Holatko, J.; Hammerschmiedt, T.; Głuchowski, A.; Mizerski, A.; Jaskulska, I.; Baltazar, T.; Kintl, A.; Jaskulski, D.; et al. Nano Zero Valent Iron (nZVI) as an Amendment for Phytostabilization of Highly Multi-PTE Contaminated Soil. Materials 2021, 14, 2559. [Google Scholar] [CrossRef] [PubMed]

| Synthesis nZVI | Preparation | Washes | Particle Size (nm) | BET-Area (m2 g−1) | Interaction or Mechanisms | References |
|---|---|---|---|---|---|---|
| Top-Down Methods | ||||||
| Mechanical | Goethite particle sizes (300–850, 125–300, 96–125, 75–96, and <75 μm) + 550 °C. | - | 80–150 | 19.9–22.8 | Precipitated of FePO4 | [28] |
| Limonite < 74 μm + °T 300, 400, 450, 500, 550, and 600 °C | - | - | 37.52–17.24 | Precipitation and adsorption | [29] | |
| Bottom-Up Methods | ||||||
| Chemical | FeCl3·6H2O + NaBH4 | Deionized (DI) water (H2O) | - | 27.65 | Adsorption and co-precipitation | [22] |
| FeCl3·H2O + NaBH4 | Three times by DI H2O and ethanol | 44 | 43.09 | Adsorption and co-precipitation | [30] | |
| FeSO4·7H2O + KBH4 | Three times by DI H2O and ethanol | 74–186 | - | Adsorption, ion exchange, and precipitation | [31] | |
| FeSO4·7H2O + KBH4 | Three times by H2O and ethanol | 30–300 | 15 | Electrostatic adsorption, ion exchange, and surface precipitation | [32] | |
| FeSO4·7H2O + NaBH4 | - | 30–80 | 20.92 | Adsorption, precipitation, and exchange | [33] | |
| FeCl3 + NaBH4 | DI H2O | 10–30 | 25 | Ligand exchange | [34] | |
| FeCl3·6H2O + NaBH4 | Three times by DI H2O | 40 | 61 | Adsorption on iron (hydr)oxides | [35] | |
| FeCl3 + NaBH4 | Three times by DI H2O | 65.4 | - | Chemical bonds | [36] | |
| FeCl3 + NaBH4 | Three times by DI H2O | 40–150 | Surface complexation, co-precipitation | [37] | ||
| Bought from Aladdin Industrial Corporation Company | - | 20−100 | 4.66 | Chemical interaction | [38] | |
| FeCl3.6H2O + NaBH4 | Three times by DI H2O and ethanol | - | - | Electrostatic attraction and co-precipitation | [39] | |
| FeSO4·7H2O + KBH4 | - | 100 | - | Physical adsorption, precipitated of Fe3(PO4)2·8H2O | [40] | |
| FeSO4·7H2O + KBH4 | DI H2O | - | 32.38 | Chemical adsorption and precipitated of Fe3(PO4)2 | [41] | |
| FeCl3·6H2O + NaBH4 | DI H2O | - | - | Electrostatic adsorption | [42] | |
| FeCl3·6H2O + NaBH4 | Ethanol | 35 | - | Electrostatic attraction and repulsion | [43] | |
| FeCl3·6H2O + KBH4 | DI H2O and anhydrous ethanol | 78 | 16.57 | Adsorption by inner-sphere surface complexes | [44] | |
| FeCl3·6H2O + NaBH4 | Three times by DI H2O | 44 | 17.32 | Adsorption on iron (hydr)oxides, co-precipitation | [45] | |
| FeCl3·6H2O + NaBH4 | Three times by DI H2O and ethanol | 13 | 32.38 | Physical adsorption and chemical adsorption | [46] | |
| FeCl3·6H2O + NaBH4 | Ethanol | 50 | 143.163 | Electrostatic attraction and co-precipitation | [47] | |
| FeSO4·7H2O + leaves of Shirazi thyme FeSO4·7H2O + pistachio green hulls | Ethylene | 40–70 | - | Physical and chemical adsorption on iron (hydr)oxides and precipitation | [48] | |
| Green biological | FeCl3 + pomegranate (GP) | - | 114 | - | Electrostatic adsorption and surface complexation | [49] |
| FeCl3 + banana (BP) | 96 | |||||
| FeCl3 + mango (MP) | 75 | |||||
| FeCl3·6H2O + black tea | Ethanol | - | - | Binding | [50] | |
| Adsorbent | Preparation Materials | Washes | Particle Size (nm) | BET-Area (m2 g−1) | Interaction Mechanisms or Reactions with Phosphate | References |
|---|---|---|---|---|---|---|
| nZVI Stabilized with Organic Molecules | ||||||
| Chitosan-nZVI | FeSO4·7H2O + KBH4 + medium molecular weight chitosan | - | 117–200 | - | Adsorption, ion exchange, and precipitation | [31] |
| CMC-nZVI | FeSO4·7H2O + NaBH4 + CMC | - | - | - | - | [33] |
| CMC-nZVI | FeCl3 + NaBH4 + polymer (CMC, PAA, PSM, and PVP) | Three times by DI H2O | 9.53 | - | Chemical bonds | [36] |
| PAA-nZVI | 106.4 | |||||
| PSM-nZVI | 106.6 | |||||
| PVP-ZVI | 108.8 | |||||
| Cation exchange resin-nZVI | FeSO4·7H2O + KBH4 + strong acid cation exchange resin | DI H2O | - | 0.13 | Chemical adsorption and precipitated of Fe3(PO4)2 | [41] |
| Starch-nZVI | FeCl3·6H2O + KBH4 + starch | DI H2O and anhydrous ethanol | 43 | 35.28 | Adsorption by inner-sphere surface complexes | [44] |
| Cellulose filter paper-nZVI | Cellulose modified with NaOH + FeCl3 anhydrous + NaBH4 | H2O and ethanol | <30 | - | Chemisorption, electrostatic attraction, and precipitated of Fe3(PO4)2·8H2O | [60] |
| SCB-nZVI) | Sugarcane bagasse + FeCl3 + KBH4 | Four times by ethyl alcohol | 150–300 | - | Electrostatic sorption and formation of inner spherical complex | [61] |
| nZVI Encapsulated | ||||||
| Alginate beads-nZVI | FeCl3·6H2O + NaBH4 + sodium alginate | - | 30–55 | - | Adsorption and chemical precipitation | [24] |
| Few-layered graphene-nZVI | Fe(NO3)3⋅9H2O + lignin + tetrahydrofuran/water | - | 5–15 | 10 | Co-precipitation and mono- and/or bidentate chemisorption interactions | [25] |
| Alginate biopolymer (Ag)-nZVI | Sodium alginate + nZVI + CaCl2 solution | - | - | - | Electrostatic interaction | [42] |
| Alginate biopolymer-nZVI | Sodium alginate + nZVI + CaCl2 solution | - | - | - | - | [43] |
| nZVI Supported on Organic Materials | ||||||
| Graphene oxides-nZVI | FeSO4·7H2O + graphitic oxide + NaBH4 | - | 15 | - | Physical adsorption, precipitated of Fe3(PO4)2·8H2O | [40] |
| Activated carbon-nZVI | FeCl3 + activated carbon +NaBH4 | Three times by ethanol | 20–60 | 88.29 | - | [62] |
| Rape straw biochar-nZVI | FeSO4 + rape straw biochar (RSBC) + NaBH4 | Three times by ethanol | 20–30 | 34.23 | Complexation, hydrogen bonding, and electrostatic interaction | [63] |
| OBW-HNO3-nZVI) | FeCl2·4H2O + ostrich bone waste-HNO3 + NaBH4 | - | 5–30 | 41.4 | Chemisorption and precipitated of Fe3(PO4)2·8H2O | [64] |
| Sulfur-nZVI@biochar | Biochar + sulfur powder + iron powder | - | - | - | Electrostatic attraction, surface chemical precipitation, hydrogen bonding, and ligand effects | [65] |
| Activated carbon/nZVI | FeCl3·6H2O +activated carbon + NaBH4 | DI H2O | <50 | 72.66 | Adsorption and co-precipitation | [66] |
| nZVI Supported on Inorganic Minerals | ||||||
| Zeolite 1-Nano | nZVI + sodium zeolite | - | - | 54.33 | Chemical adsorption and precipitated of KFeH14(PO4)8·4H2O | [46] |
| Zeolite 2-Nano | FeSO4 + sodium zeolite + nZVI | - | - | 29.01 | ||
| Bentonite-nZVI | Bentonite + iron + green tea | Ethanol | 8–30 | 32.54 | Chemisorption | [54] |
| Bentonite-nZVI | FeSO4·7H2O + leaves of green tea + natural bentonite | Ethylene | 40–60 | - | Inner-sphere binding and covalent bonds | [59] |
| Sepiolite-nZVI | FeCl3 + acid modified sepiolite + NaBH4 | Three times by ethanol | 20–70 | - | Electrostatic interaction and co-precipitation | [67] |
| Bio-apatite-nZVI | Bio-apatite + FeCl3·6H2O + NaBH4 | Ethanol | 20 to 60 | 109 | Fe3(PO4)2·8H2O | [68] |
| nZVI Bimetallic | ||||||
| FeCl3·6H2O + CuCl2 + NaBH4 | FeCl3·6H2O + CuCl2 + NaBH4 | Three times by DI H2O | 24 | 32.4 | Adsorption on iron (hydr)oxides, co-precipitation | [45] |
| Chelating resin DOW3N-Fe/Cu (D-Fe/Cu) | Fe2(SO4)3 + CuCl2·2H2O + NaBH4 + chelating resin DOW3N | DI H2O and anhydrous ethanol | - | - | Reduction, adsorption, co-precipitation, and a newly formed adsorbent-polymeric ligand exchanger | [69] |
| Chelating resin DOW3N Fe/Ni (D-Fe/Ni) | Fe2(SO4)3 + NiCl2·6H2O + NaBH4 + chelating resin DOW3N | - | - | |||
| Zeolite-Fe/Ni (Z-Fe/Ni) | FeCl3·6H2O + NiCl2·6H2O + acid-activated zeolite + NaBH4 | Three times by H2O and ethanol | 20–30 | 59.2 | Reduction and complexation | [70] |
| Adsorbent | Best-Fitted Isotherm Model | °T | pH | IC (mg L−1) | IC (µM) | Removal Efficiency (%) | qmax (mg g−1) | PC (mg g−1 µM−1) | References |
|---|---|---|---|---|---|---|---|---|---|
| Freundlich | 25 | - | 1000 | 32,289.3 | 94 | 245.6 | 0.127 | [22] | |
| Langmuir | 25 | 7 | 1000 | 32,289.3 | 5.4 | 54.3 | 0.002 | [30] | |
| nZVI | Langmuir | 25 | 5 | 175 | 5650.6 | 60.7 | 437.4 | 0.197 | [31] |
| Langmuir | 7 | 175 | 5650.6 | 24.4 | 168.5 | 0.039 | |||
| Langmuir | - | 4.5 | 500 | 16,144.7 | 29.9 | 523 | 0.046 | [32] | |
| Langmuir | 6.5 | 500 | 16,144.7 | 23.5 | 412 | 0.033 | |||
| - | 6 | 90 | 2906.0 | 51.4 | 115.6 | 0.082 | [33] | ||
| - | 25 | 7 | 1000 | 32,289.3 | 7.6 | 61.1 | 0.002 | [36] | |
| Langmuir | 20 | 7 | 6 | 193.7 | 88 | 32.4 | 1.393 | [38] | |
| Langmuir | 25 | 7 | 100 | 3228.9 | 76.8 | 76.8 | 0.103 | [39] | |
| Freundlich | 20 | - | 20 | 645.8 | 39.1 | 15.6 | 0.04 | [40] | |
| Langmuir | 10 | 4 | 440 | 14,207.3 | 56.2 | 247.3 | 0.04 | [44] | |
| Nanocomposite | |||||||||
| Organic | |||||||||
| Chitosan-nZVI | Langmuir | 25 | 5 | 175 | 5650.6 | 49.6 | 289.2 | 0.101 | [31] |
| 7 | 26.1 | 152.3 | 0.036 | ||||||
| CMC-nZVI | Langmuir | 25 | 7 | 1000 | 32,289.3 | 7.1 | 56.8 | 0.002 | [36] |
| PAA-nZVI | 9.75 | 78 | 0.003 | ||||||
| PSM-nZVI | 7.5 | 60 | 0.002 | ||||||
| PVP-nZVI | 7.3 | 58.4 | 0.002 | ||||||
| Pistachio green hulls-nZVI | Redlich–Peterson | room | 5 | 300 | 9686.8 | 23.61 | 29.3 | 0.004 | [48] |
| Shirazi thyme leaf-nZVI | 9686.8 | 35.79 | 40.5 | 0.007 | |||||
| SCB/nZVI | Langmuir | 5.3 | 200 | 6457.9 | 41.04 | 205.2 | 0.216 | [61] | |
| Encapsulated | |||||||||
| Alginate beads-nZVI | - | - | - | 10 | 322.9 | 62.4 | 0.312 | 0.003 | [24] |
| Inorganic | |||||||||
| Graphene oxides-nZVI | Freundlich | 20 | - | 20 | 645.8 | 36.8 | 14.7 | 0.036 | [40] |
| Starch-nZVI | Langmuir | 10 | 4 | 440 | 14,207.3 | 73.2 | 322.4 | 0.085 | [44] |
| Bentonite-nZVI | Sips | room | - | 900 | 29,060.4 | 32.6 | 58.6 | 0.003 | [54] |
| Bentonite-nZVI | Redlich–Peterson | - | - | 500 | 16,144.7 | 27.6 | 27.6 | 0.002 | [59] |
| Biochar-nZVI | Sips | 25 | 7 | 60 | 1937.4 | 40.5 | 12.1 | 0.011 | [63] |
| Ostrich bone waste-HNO3-nZVI | Langmuir | 25 | 5 | 1000 | 32,289.3 | 32.6 | 326 | 0.015 | [64] |
| Sepiolite-nZVI | Freundlich | - | 4.5 | 25 | 807.2 | 99.4 | 16 | 3.477 | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suazo-Hernández, J.; Sepúlveda, P.; Cáceres-Jensen, L.; Castro-Rojas, J.; Poblete-Grant, P.; Bolan, N.; Mora, M.d.l.L. nZVI-Based Nanomaterials Used for Phosphate Removal from Aquatic Systems. Nanomaterials 2023, 13, 399. https://doi.org/10.3390/nano13030399
Suazo-Hernández J, Sepúlveda P, Cáceres-Jensen L, Castro-Rojas J, Poblete-Grant P, Bolan N, Mora MdlL. nZVI-Based Nanomaterials Used for Phosphate Removal from Aquatic Systems. Nanomaterials. 2023; 13(3):399. https://doi.org/10.3390/nano13030399
Chicago/Turabian StyleSuazo-Hernández, Jonathan, Pamela Sepúlveda, Lizethly Cáceres-Jensen, Jorge Castro-Rojas, Patricia Poblete-Grant, Nanthi Bolan, and María de la Luz Mora. 2023. "nZVI-Based Nanomaterials Used for Phosphate Removal from Aquatic Systems" Nanomaterials 13, no. 3: 399. https://doi.org/10.3390/nano13030399
APA StyleSuazo-Hernández, J., Sepúlveda, P., Cáceres-Jensen, L., Castro-Rojas, J., Poblete-Grant, P., Bolan, N., & Mora, M. d. l. L. (2023). nZVI-Based Nanomaterials Used for Phosphate Removal from Aquatic Systems. Nanomaterials, 13(3), 399. https://doi.org/10.3390/nano13030399