Tailoring Heat Transfer and Bactericidal Response in Multifunctional Cotton Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
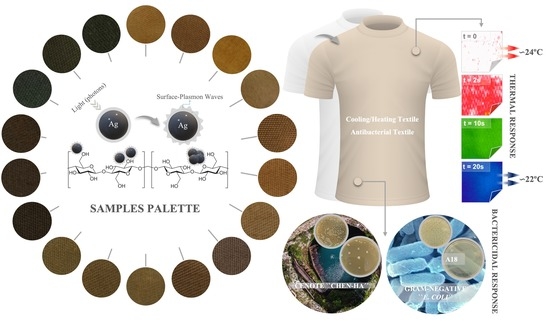
2.2. Functionalization and Design of “Smart” Cotton Fabrics
2.2.1. Immobilization In Situ of AgNPs
2.2.2. Characterization of AgNP-Coated Cotton Fabrics
2.2.3. AgNP-Coated Release Kinetics
2.3. Temperature Response Measurements
2.4. Bactericidal Response Measurements
2.4.1. Assay with Natural Samples
2.4.2. Assay with Reference Samples
3. Results and Discussion
3.1. Functionalization and Design of “Smart” Cotton Fabrics
3.1.1. Surface Morphology of Coated Fibers
3.1.2. Surface Chemistry of Coated Fibers
3.1.3. Colorimetric Analysis of Coated Fibers
3.1.4. Thermal Analysis of Coated Fibers
3.1.5. AgNP-Coated Release Kinetics
3.2. Temperature Response Measurements
3.3. Bactericidal Response Measurements
3.3.1. Assay with Natural Samples
3.3.2. Assay with Reference Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ma, Z.; Yang, Z.; Gao, Q.; Bao, G.; Valiei, A.; Yang, F.; Huo, R.; Wang, C.; Song, G.; Ma, D.; et al. Bioinspired tough gel sheath for robust and versatile surface functionalization. Sci. Adv. 2021, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, P.S.; Bhattacharya, C.; Afewerki, S.; Langer, R.S. Smart Biomaterials: Recent Advances and Future Directions. ACS Biomater. Sci. Eng. 2018, 4, 3809–3817. [Google Scholar] [CrossRef] [PubMed]
- Amukarimi, S.; Ramakrishna, S.; Mozafari, M. Smart biomaterials—A proposed definition and overview of the field. Curr. Opin. Biomed. Eng. 2021, 19, 100311. [Google Scholar] [CrossRef]
- Coyle, S.; Wu, Y.; Lau, K.-T.; De Rossi, D.; Wallace, G.; Diamond, D. Smart Nanotextiles: A Review of Materials and Applications. MRS Bull. 2007, 32, 434–442. [Google Scholar] [CrossRef] [Green Version]
- Madan, C. Fabric wearable allows for adaptive personal heat management. MRS Bull. 2022, 47, 213. [Google Scholar] [CrossRef]
- Sharma, J.; Lizu, M.; Stewart, M.; Zygula, K.; Lu, Y.; Chauhan, R.; Yan, X.; Guo, Z.; Wujcik, E.K.; Wei, S. Multifunctional Nanofibers towards Active Biomedical Therapeutics. Polymers 2015, 7, 186–219. [Google Scholar] [CrossRef] [Green Version]
- Sarvalkar, P.D.; Barawkar, S.D.; Karvekar, O.S.; Patil, P.D.; Prasad, S.R.; Sharma, K.K.; Prasad, N.R.; Vhatkar, R.S. A review on multifunctional nanotechnological aspects in modern textile. J. Text. Inst. 2022, 8, 1–18. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 22. [Google Scholar] [CrossRef]
- Chandika, P.; Khan, F.; Heo, S.-Y.; Kim, Y.-M.; Yi, M.; Jung, W.-K. Enhanced wound-healing capability with inherent antimicrobial activities of usnic acid incorporated poly(ε-caprolactone)/decellularized extracellular matrix nanofibrous scaffold. Biomater. Adv. 2022, 140, 213046. [Google Scholar] [CrossRef]
- Chen, Q.; Li, S.; Zhao, W.; Zhao, C. A rapid-triggered approach towards antibacterial hydrogel wound dressing with synergic photothermal and sterilization profiles. Biomater. Adv. 2022, 138, 212873. [Google Scholar] [CrossRef]
- Atiyeh, B.S.; Costagliola, M.; Hayek, S.N.; Dibo, S.A. Effect of silver on burn wound infection control and healing: Review of the literature. Burns 2007, 33, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Velnar, T.; Bailey, T.; Smrkolj, V. The Wound Healing Process: An Overview of the Cellular and Molecular Mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Rojas, O.J. (Ed.) Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Springer International Publishing: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Liang, C.; Ma, T.; Guo, Y.; Kong, J.; Gu, J.; Chen, M.; Zhu, J. A review on thermally conductive polymeric composites: Classification, measurement, model and equations, mechanism and fabrication methods. Adv. Compos. Hybrid Mater. 2018, 1, 207–230. [Google Scholar] [CrossRef]
- Fievet, F.; Lagier, J.; Figlarz, M. Preparing Monodisperse Metal Powders in Micrometer and Submicrometer Sizes by the Polyol Process. MRS Bull. 1989, 14, 29–34. [Google Scholar] [CrossRef]
- Fievet, F.; Lagier, J.; Blin, B.; Beaudoin, B.; Figlarz, M. Homogeneous and heterogeneous nucleations in the polyol process for the preparation of micron and submicron size metal particles. Solid State Ion. 1989, 32-33, 198–205. [Google Scholar] [CrossRef]
- Ammar, S.; Fiévet, F. Polyol Synthesis: A Versatile Wet-Chemistry Route for the Design and Production of Functional Inorganic Nanoparticles. Nanomaterials 2020, 10, 1217. [Google Scholar] [CrossRef]
- Ducamp-Sanguesa, C.; Herrera-Urbina, R.; Figlarz, M. Synthesis and characterization of fine and monodisperse silver particles of uniform shape. J. Solid State Chem. 1992, 100, 272–280. [Google Scholar] [CrossRef]
- Zeng, X.; Zhou, B.; Gao, Y.; Wang, C.; Li, S.; Yeung, C.Y.; Wen, W. Structural dependence of silver nanowires on polyvinyl pyrrolidone (PVP) chain length. Nanotechnology 2014, 25, 495601. [Google Scholar] [CrossRef]
- Jiang, X.C.; Chen, W.M.; Chen, C.Y.; Xiong, S.X.; Yu, A. Role of Temperature in the Growth of Silver Nanoparticles Through a Synergetic Reduction Approach. Nanoscale Res. Lett. 2010, 6, 32–39. [Google Scholar] [CrossRef] [Green Version]
- Gartner, I.T.E.; Jayaraman, A. Modeling and Simulations of Polymers: A Roadmap. Macromolecules 2019, 52, 755–786. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.; Li, L.; Jiang, X.; Liu, F.; Liu, Q.; Liu, X. Construction of silver-cotton carbon fiber sensing interface and study on the protective effect of antioxidants on hypoxia-induced cell damage. Microchem. J. 2020, 159, 105345. [Google Scholar] [CrossRef]
- Atta, A.M.; Abomelka, H.M. Multifunctional finishing of cotton fibers using silver nanoparticles via microwave-assisted reduction of silver alkylcarbamate. Mater. Chem. Phys. 2020, 260, 124137. [Google Scholar] [CrossRef]
- Nam, S.; Baek, I.-S.; Hillyer, M.B.; He, Z.; Barnaby, J.Y.; Condon, B.D.; Kim, M.S. Thermosensitive textiles made from silver nanoparticle-filled brown cotton fibers. Nanoscale Adv. 2022, 4, 3725–3736. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Balankura, T.; Fichthorn, K.A.; Rioux, R.M. Revisiting the Polyol Synthesis of Silver Nanostructures: Role of Chloride in Nanocube Formation. ACS Nano 2019, 13, 1849–1860. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Hodges, C.; Biggs, S.R.; Cayre, O.J.; Harbottle, D. Polymer Molecular Weight Dependence on Lubricating Particle–Particle Interactions. Ind. Eng. Chem. Res. 2018, 57, 2131–2138. [Google Scholar] [CrossRef]
- Skrabalak, E.S.; Au, L.; Li, X.; Xia, Y. Facile synthesis of Ag nanocubes and Au nanocages. Nat. Protoc. 2007, 2, 2182–2190. [Google Scholar] [CrossRef]
- Fiévet, F.; Ammar-Merah, S.; Brayner, R.; Chau, F.; Giraud, M.; Mammeri, F.; Peron, J.; Piquemal, J.-Y.; Sicard, L.; Viau, G. The polyol process: A unique method for easy access to metal nanoparticles with tailored sizes, shapes and compositions. Chem. Soc. Rev. 2018, 47, 5187–5233. [Google Scholar] [CrossRef]
- Bonet, F.; Tekaia-Elhsissen, K.; Sarathy, K.V. Study of interaction of ethylene glycol/PVP phase on noble metal powders prepared by polyol process. Bull. Mater. Sci. 2000, 23, 165–168. [Google Scholar] [CrossRef]
- Kyrychenko, A.; Korsun, O.M.; Gubin, I.I.; Kovalenko, S.M.; Kalugin, O.N. Atomistic Simulations of Coating of Silver Nanoparticles with Poly(vinylpyrrolidone) Oligomers: Effect of Oligomer Chain Length. J. Phys. Chem. C 2015, 119, 7888–7899. [Google Scholar] [CrossRef]
- Wiley, B.; Sun, Y.; Mayers, B.; Xia, Y. Shape-Controlled Synthesis of Metal Nanostructures: The Case of Silver. Chem.-A Eur. J. 2005, 11, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xia, Y. Shape-Controlled Synthesis of Gold and Silver Nanoparticles. Science 2002, 298, 2176–2179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatemeh, K.; Javad, M.M.; Samaneh, K. The effect of silver nanoparticles on composite shear bond strength to dentin with different adhesion protocols. J. Appl. Oral Sci. 2017, 25, 367–373. [Google Scholar] [CrossRef]
- Kim, D.; Jeong, S.; Moon, J. Synthesis of silver nanoparticles using the polyol process and the influence of precursor injection. Nanotechnology 2006, 17, 4019–4024. [Google Scholar] [CrossRef]
- Shameli, K.; Bin Ahmad, M.; Zamanian, A.; Sangpour, P.; Shabanzadeh, P.; Abdollahi, Y.; Zargar, M. Green biosynthesis of silver nanoparticles using Curcuma longa tuber powder. Int. J. Nanomed. 2012, 7, 5603–5610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiley, J.H.; Atalla, R.H. Band assignments in the raman spectra of celluloses. Carbohydr. Res. 1987, 160, 113–129. [Google Scholar] [CrossRef]
- Ko, H.; Singamaneni, S.; Tsukruk, V.V. Nanostructured Surfaces and Assemblies as SERS Media. Small 2008, 4, 1576–1599. [Google Scholar] [CrossRef]
- Lu, X.; Rycenga, M.; Skrabalak, S.E.; Wiley, B.; Xia, Y. Chemical Synthesis of Novel Plasmonic Nanoparticles. Annu. Rev. Phys. Chem. 2009, 60, 167–192. [Google Scholar] [CrossRef]
- Xia, Y.; Halas, N.J. Shape-Controlled Synthesis and Surface Plasmonic Properties of Metallic Nanostructures. MRS Bull. 2005, 30, 338–348. [Google Scholar] [CrossRef]
- Dubas, S.T.; Kumlangdudsana, P.; Potiyaraj, P. Layer-by-layer deposition of antimicrobial silver nanoparticles on textile fibers. Colloids Surf. A Physicochem. Eng. Asp. 2006, 289, 105–109. [Google Scholar] [CrossRef]
- Božič, M.; Kokol, V. Ecological alternatives to the reduction and oxidation processes in dyeing with vat and sulphur dyes. Dyes Pigments 2008, 76, 299–309. [Google Scholar] [CrossRef]
- Rycenga, M.; Cobley, C.M.; Zeng, J.; Li, W.; Moran, C.H.; Zhang, Q.; Qin, D.; Xia, Y. Controlling the Synthesis and Assembly of Silver Nanostructures for Plasmonic Applications. Chem. Rev. 2011, 111, 3669–3712. [Google Scholar] [CrossRef] [Green Version]
- Luo, W.; Hu, W.; Xiao, S. Size Effect on the Thermodynamic Properties of Silver Nanoparticles. J. Phys. Chem. C 2008, 112, 2359–2369. [Google Scholar] [CrossRef]
- Montazer, M.; Nia, Z.K. Conductive nylon fabric through in situ synthesis of nano-silver: Preparation and characterization. Mater. Sci. Eng. C 2015, 56, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, J.; Rana, S. Thermal degradation and morphological studies on cotton cellulose modified with various arylphosphorodichloridites. Polym. Int. 2004, 53, 995–1002. [Google Scholar] [CrossRef]
- Ma, R.; Levard, C.; Marinakos, S.M.; Cheng, Y.; Liu, J.; Michel, F.M.; Brown, J.G.E.; Lowry, G.V. Size-Controlled Dissolution of Organic-Coated Silver Nanoparticles. Environ. Sci. Technol. 2011, 46, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Wuhrmann, K.; Zobrist, F. Untersuchungen über die bakterizide Wirkung von Silber in Wasser. Aquat. Sci. 1958, 20, 218–254. [Google Scholar] [CrossRef]
- Joyce-Wöhrmann, R.M.; Hentschel, T.; Münstedt, H. Thermoplastic Silver-Filled Polyurethanes for Antimicrobial Catheters. Adv. Eng. Mater. 2000, 2, 380–386. [Google Scholar] [CrossRef]
- Fernández, A.; Soriano, E.; Hernández-Muñoz, P.; Gavara, R. Migration of Antimicrobial Silver from Composites of Polylactide with Silver Zeolites. J. Food Sci. 2010, 75, E186–E193. [Google Scholar] [CrossRef]
- Gutierrez-Juarez, G.; Acosta-Avalos, D.; Medina, R.; Vargas-Luna, M.; Alvarado-Gil, J.J. Metrological aspects of thermal relaxation technique by radiation loss for volumetric heat capacity measurements. Eur. Phys. J. Spéc. Top. 2008, 153, 171–173. [Google Scholar] [CrossRef]
- Pech-May, N.W.; Cifuentes, Á.; Mendioroz, A.; Oleaga, A.; Salazar, A. Simultaneous measurement of thermal diffusivity and effusivity of solids using the flash technique in the front-face configuration. Meas. Sci. Technol. 2015, 26, 085017. [Google Scholar] [CrossRef] [Green Version]
- Almond, D.; Patel, P. Photothermal Science and Techniques, 1st ed.; Chapman & Hall: London, UK, 1996; ISBN-10: 0412578808. [Google Scholar]
- Michael Hollas, J. Modern Spectroscopy, 4th ed.; Wiley (John Wiley & Sons, Ltd.): West Sussex, UK, 2004; ISBN 978-0-470-84416-8. [Google Scholar]
- Mayerhöfer, T.G.; Pipa, A.V.; Popp, J. Beer’s Law-Why Integrated Absorbance Depends Linearly on Concentration. ChemPhysChem 2019, 20, 2748–2753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnepp, O. Theory for the Infrared Absorption Intensities of the Lattice Vibrations of Molecular Solids. J. Chem. Phys. 1967, 46, 3983–3990. [Google Scholar] [CrossRef]
- Hussein, A.E.; Zagho, M.M.; Nasrallah, G.K.; Elzatahry, A.A. Recent advances in functional nanostructures as cancer photothermal therapy. Int. J. Nanomed. 2018, 13, 2897–2906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sönnichsen, C.; Reinhard, B.M.; Liphardt, J.; Alivisatos, P. A molecular ruler based on plasmon coupling of single gold and silver nanoparticles. Nat. Biotechnol. 2005, 23, 741–745. [Google Scholar] [CrossRef] [Green Version]
- Coronado, E.A.; Encina, E.R.; Stefani, F.D. Optical properties of metallic nanoparticles: Manipulating light, heat and forces at the nanoscale. Nanoscale 2011, 3, 4042–4059. [Google Scholar] [CrossRef] [PubMed]
- Prodan, E.; Radloff, C.; Halas, N.J.; Nordlander, P. A Hybridization Model for the Plasmon Response of Complex Nanostructures. Science 2003, 302, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Encina, E.R.; Coronado, E.A. Plasmon Coupling in Silver Nanosphere Pairs. J. Phys. Chem. C 2010, 114, 3918–3923. [Google Scholar] [CrossRef]
- Cha, H.; Lee, D.; Yoon, J.H.; Yoon, S. Plasmon coupling between silver nanoparticles: Transition from the classical to the quantum regime. J. Colloid Interface Sci. 2016, 464, 18–24. [Google Scholar] [CrossRef]
- Salazar, A. On thermal diffusivity. Eur. J. Phys. 2003, 24, 351–358. [Google Scholar] [CrossRef]
- Escobedo-Hinojosa, W.; Pardo-López, L. Analysis of bacterial metagenomes from the Southwestern Gulf of Mexico for pathogens detection. Pathog. Dis. 2017, 75, ftx058. [Google Scholar] [CrossRef] [PubMed]
- Smart, P.L.; Beddows, P.A.; Coke, J.; Doerr, S.; Smith, S.; Whitaker, F.F. Cave development on the Caribbean coast of the Yucatan Peninsula, Quintana Roo, Mexico. Spec. Pap. Geol. Soc. Am. 2006, 404, 105–128. [Google Scholar] [CrossRef] [Green Version]
- Bauer-Gottwein, P.; Gondwe, B.R.N.; Charvet, G.; Marín, L.E.; Rebolledo-Vieyra, M.; Merediz-Alonso, G. Review: The Yucatán Peninsula karst aquifer, Mexico. Hydrogeol. J. 2011, 19, 507–524. [Google Scholar] [CrossRef]
- González-Herrera, R.; Sánchez-Y.-Pinto, I.; Gamboa-Vargas, J. Groundwater-flow modeling in the Yucatan karstic aquifer, Mexico. Hydrogeol. J. 2002, 10, 539–552. [Google Scholar] [CrossRef]
- Huang, L.; Bae, H.; Young, C.; Pain, A.J.; Martin, J.B.; Ogram, A. Campylobacterota dominate the microbial communities in a tropical karst subterranean estuary, with implications for cycling and export of nitrogen to coastal waters. Environ. Microbiol. 2021, 23, 6749–6763. [Google Scholar] [CrossRef]
- Bapat, R.A.; Chaubal, T.V.; Joshi, C.P.; Bapat, P.R.; Choudhury, H.; Pandey, M.; Gorain, B.; Kesharwani, P. An overview of application of silver nanoparticles for biomaterials in dentistry. Mater. Sci. Eng. C 2018, 91, 881–898. [Google Scholar] [CrossRef]
- Ahmad, S.A.; Das, S.S.; Khatoon, A.; Ansari, M.T.; Afzal, M.; Hasnain, S.; Nayak, A.K. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Shchekin, A.K.; Rusanov, A.I. Generalization of the Gibbs–Kelvin–Köhler and Ostwald–Freundlich equations for a liquid film on a soluble nanoparticle. J. Chem. Phys. 2008, 129, 154116. [Google Scholar] [CrossRef] [Green Version]
- Shanmuganathan, R.; MubarakAli, D.; Prabakar, D.; Muthukumar, H.; Thajuddin, N.; Kumar, S.S.; Pugazhendhi, A. An enhancement of antimicrobial efficacy of biogenic and ceftriaxone-conjugated silver nanoparticles: Green approach. Environ. Sci. Pollut. Res. 2017, 25, 10362–10370. [Google Scholar] [CrossRef]
- Khorrami, S.; Najafabadi, F.J.; Zarepour, A.; Zarrabi, A. Is Astragalus gossypinus Honey a Natural Antibacterial and Cytotoxic Agent? An Investigation on A. gossypinus Honey Biological Activity and Its Green Synthesized Silver Nanoparticles. Bionanoscience 2019, 9, 603–610. [Google Scholar] [CrossRef]
- Eby, D.M.; Schaeublin, N.M.; Farrington, K.E.; Hussain, S.M.; Johnson, G.R. Lysozyme Catalyzes the Formation of Antimicrobial Silver Nanoparticles. ACS Nano 2009, 3, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.; Lam, K.; Hansen, D.; Burrell, R. Efficacy of topical silver against fungal burn wound pathogens. Am. J. Infect. Control. 1999, 27, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Yakabe, Y.; Sano, T.; Ushio, H.; Yasunaga, T. Kinetic studies of the interaction between silver ion and deoxyribonucleic acid. Chem. Lett. 1980, 9, 373–376. [Google Scholar] [CrossRef]
- Chouhan, S.; Guleria, S. Green synthesis of AgNPs using Cannabis sativa leaf extract: Characterization, antibacterial, anti-yeast and α-amylase inhibitory activity. Mater. Sci. Energy Technol. 2020, 3, 536–544. [Google Scholar] [CrossRef]
- Liao, C.; Li, Y.; Tjong, S.C. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef] [Green Version]
- Khorrami, S.; Zarrabi, A.; Khaleghi, M.; Danaei, M.; Mozafari, M.R. Selective cytotoxicity of green synthesized silver nanoparticles against the MCF-7 tumor cell line and their enhanced antioxidant and antimicrobial properties. Int. J. Nanomed. 2018, 13, 8013–8024. [Google Scholar] [CrossRef] [Green Version]
- Ratte, H.T. Bioaccumulation and toxicity of silver compounds: A review. Environ. Toxicol. Chem. 1999, 18, 89–108. [Google Scholar] [CrossRef]
- Bottero, J.-Y.; Auffan, M.; Rose, J.; Mouneyrac, C.; Botta, C.; Labille, J.; Masion, A.; Thill, A.; Chaneac, C. Manufactured metal and metal-oxide nanoparticles: Properties and perturbing mechanisms of their biological activity in ecosystems. C. R. Geosci. 2011, 343, 168–176. [Google Scholar] [CrossRef]
- Das, S.S.; Alkahtani, S.; Bharadwaj, P.; Ansari, M.T.; Alkahtani, M.D.; Pang, Z.; Hasnain, S.; Nayak, A.K.; Aminabhavi, T.M. Molecular insights and novel approaches for targeting tumor metastasis. Int. J. Pharm. 2020, 585, 119556. [Google Scholar] [CrossRef]
- Levard, C.; Hotze, E.M.; Lowry, G.V.; Brown, J.G.E. Environmental Transformations of Silver Nanoparticles: Impact on Stability and Toxicity. Environ. Sci. Technol. 2012, 46, 6900–6914. [Google Scholar] [CrossRef]









| Sample | (s) | AgNPs Fixed (%) | Integrated Absorbance (320–1000 nm) |
|---|---|---|---|
| A01 | 38 | 0.40 | 15.2 |
| A02 | 43 | 0.61 | 19.5 |
| A03 | 41 | 1.28 | 22.5 |
| A16 | 21 | 4.91 | 44.1 |
| A17 | 25 | 4.03 | 46.4 |
| A18 | 24 | 1.43 | 49.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado, L.P.; Franco-Bacca, A.P.; Cervantes-Alvarez, F.; Ortiz-Vazquez, E.; Ramon-Sierra, J.M.; Rejon, V.; Aguirre-Macedo, M.L.; Alvarado-Gil, J.J.; Rodríguez-Gattorno, G. Tailoring Heat Transfer and Bactericidal Response in Multifunctional Cotton Composites. Nanomaterials 2023, 13, 463. https://doi.org/10.3390/nano13030463
Delgado LP, Franco-Bacca AP, Cervantes-Alvarez F, Ortiz-Vazquez E, Ramon-Sierra JM, Rejon V, Aguirre-Macedo ML, Alvarado-Gil JJ, Rodríguez-Gattorno G. Tailoring Heat Transfer and Bactericidal Response in Multifunctional Cotton Composites. Nanomaterials. 2023; 13(3):463. https://doi.org/10.3390/nano13030463
Chicago/Turabian StyleDelgado, Lilian Pérez, Adriana Paola Franco-Bacca, Fernando Cervantes-Alvarez, Elizabeth Ortiz-Vazquez, Jesús Manuel Ramon-Sierra, Victor Rejon, María Leopoldina Aguirre-Macedo, Juan José Alvarado-Gil, and Geonel Rodríguez-Gattorno. 2023. "Tailoring Heat Transfer and Bactericidal Response in Multifunctional Cotton Composites" Nanomaterials 13, no. 3: 463. https://doi.org/10.3390/nano13030463
APA StyleDelgado, L. P., Franco-Bacca, A. P., Cervantes-Alvarez, F., Ortiz-Vazquez, E., Ramon-Sierra, J. M., Rejon, V., Aguirre-Macedo, M. L., Alvarado-Gil, J. J., & Rodríguez-Gattorno, G. (2023). Tailoring Heat Transfer and Bactericidal Response in Multifunctional Cotton Composites. Nanomaterials, 13(3), 463. https://doi.org/10.3390/nano13030463