Silver Nanoshells with Optimized Infrared Optical Response: Synthesis for Thin-Shell Formation, and Optical/Thermal Properties after Embedding in Polymeric Films
Abstract
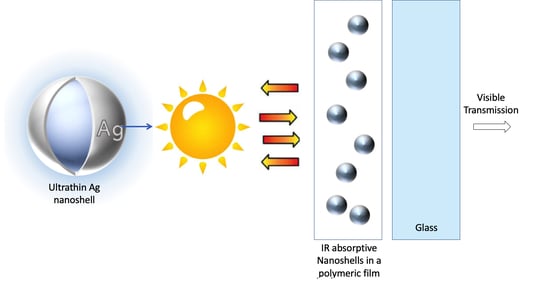
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.2.1. Synthesis of the Silica Seeds
2.2.2. Regrowth of the Silica Seeds
2.2.3. Additional Regrowth of the Silica Particles
2.2.4. Sn-Sensitization of Silica Particles
2.2.5. Preparation of Tollens’ Reagent
2.2.6. Synthesis of Ag Seeds on Sn-Functionalized Silica Particles
2.2.7. Formation of the Thin Silver Shell
2.3. Composite-Film Preparation
2.4. Characterization
3. Results
3.1. Silver-Seeded Silica Particles
3.2. Ultrathin Silver Nanoshell
3.3. Synthesis Mechanism
3.4. The Effects of Various Synthetic Parameters
3.5. Optical and Thermal Properties of Dispersed Ag Nanoshells in Polymer Films on Glass
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jankiewicz, B.; Jamiola, D.; Choma, J.; Jaroniec, M. Silica–metal core–shell nanostructures. Adv. Colloid Interface Sci. 2012, 170, 28–47. [Google Scholar] [CrossRef] [PubMed]
- Hao, E.; Li, S.; Bailey, R.C.; Zou, S.; Schatz, G.C.; Hupp, J.T. Optical Properties of Metal Nanoshells. J. Phys. Chem. B 2004, 108, 1224–1229. [Google Scholar] [CrossRef]
- Oldenburg, S.; Averitt, R.; Westcott, S.; Halas, N. Nanoengineering of optical resonances. Chem. Phys. Lett. 1998, 288, 243–247. [Google Scholar] [CrossRef]
- Bardhan, R.; Lal, S.; Joshi, A.; Halas, N.J. Theranostic Nanoshells: From Probe Design to Imaging and Treatment of Cancer. Accounts Chem. Res. 2011, 44, 936–946. [Google Scholar] [CrossRef]
- Henderson, L.; Neumann, O.; Kadria-Vili, Y.; Gerislioglu, B.; Bankson, J.; Nordlander, P.; Halas, N.J. Plasmonic gadolinium oxide nanomatryoshkas: Bifunctional magnetic resonance imaging enhancers for photothermal cancer therapy. PNAS Nexus 2022, 1, 1. [Google Scholar] [CrossRef]
- Jackson, J.B.; Halas, N.J. Silver Nanoshells: Variations in Morphologies and Optical Properties. J. Phys. Chem. B 2001, 105, 2743–2746. [Google Scholar] [CrossRef]
- Kneipp, K.; Kneipp, H.; Kneipp, J. Surface-Enhanced Raman Scattering in Local Optical Fields of Silver and Gold Nanoaggregates From Single-Molecule Raman Spectroscopy to Ultrasensitive Probing in Live Cells. Accounts Chem. Res. 2006, 39, 443–450. [Google Scholar] [CrossRef]
- Besteiro, L.V.; Kong, X.-T.; Wang, Z.; Rosei, F.; Govorov, A.O. Plasmonic Glasses and Films Based on Alternative Inexpensive Materials for Blocking Infrared Radiation. Nano Lett. 2018, 18, 3147–3156. [Google Scholar] [CrossRef]
- Liu, T.; Li, D.; Yang, D.; Jiang, M. An improved seed-mediated growth method to coat complete silver shells onto silica spheres for surface-enhanced Raman scattering. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 387, 17–22. [Google Scholar] [CrossRef]
- Flores, J.C.; Torres, V.; Popa, M.; Crespo, D.; Calderón-Moreno, J.M. Variations in morphologies of silver nanoshells on silica spheres. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 330, 86–90. [Google Scholar] [CrossRef]
- Kang, H.; Yang, J.-K.; Noh, M.S.; Jo, A.; Jeong, S.; Lee, M.; Lee, S.; Chang, H.; Lee, H.; Jeon, S.-J.; et al. One-step synthesis of silver nanoshells with bumps for highly sensitive near-IR SERS nanoprobes. J. Mater. Chem. B 2014, 2, 4415–4421. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.G.; Kang, H.; Choi, Y.-S.; Cho, Y.; Lee, M.; Lee, H.-Y.; Lee, Y.-S.; Jeong, D.H. Effect of Alkylamines on Morphology Control of Silver Nanoshells for Highly Enhanced Raman Scattering. ACS Appl. Mater. Interfaces 2019, 11, 8374–8381. [Google Scholar] [CrossRef]
- Yang, J.-K.; Kang, H.; Lee, H.; Jo, A.; Jeong, S.; Jeon, S.-J.; Kim, H.-I.; Lee, H.-Y.; Jeong, D.H.; Kim, J.-H.; et al. Single-Step and Rapid Growth of Silver Nanoshells as SERS-Active Nanostructures for Label-Free Detection of Pesticides. ACS Appl. Mater. Interfaces 2014, 6, 12541–12549. [Google Scholar] [CrossRef]
- Dong, A.G.; Wang, Y.J.; Tang, Y.; Ren, N.; Yang, W.L.; Gao, Z. Fabrication of compact silver nanoshells on polystyrene spheres through electrostatic attraction. Chem. Commun. 2002, 350–351. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wan, J.; Wei, X.; Liu, Y.; Huang, J.; Sun, X.; Zhang, R.; Gurav, D.D.; Vedarethinam, V.; Li, Y.; et al. Plasmonic silver nanoshells for drug and metabolite detection. Nat. Commun. 2017, 8, 220. [Google Scholar] [CrossRef]
- Pan, K.-Y.; Liang, Y.-F.; Pu, Y.-C.; Hsu, Y.-J.; Yeh, J.-W.; Shih, H.C. Studies on the photocatalysis of core-shelled SiO2–Ag nanospheres by controlled surface plasmon resonance under visible light. Appl. Surf. Sci. 2014, 311, 399–404. [Google Scholar] [CrossRef]
- Ray, N.K.; Patel, H.; Muñoz-Espí, R.; Landfester, K.; Gundabala, V. Synthesis of silver-coated polystyrene latex through in situ metal reduction. MRS Commun. 2022, 12, 952–958. [Google Scholar] [CrossRef]
- Chen, W.-S.; Chen, H.-R.; Lee, C.-H. The Photocatalytic Performance of Ag-Decorated SiO2 Nanoparticles (NPs) and Binding Ability between Ag NPs and Modifiers. Coatings 2022, 12, 146. [Google Scholar] [CrossRef]
- Maw, S.S.; Watanabe, S.; Miyahara, M.T. Multiple Roles of Polyethylenimine during Synthesis of 10 nm Thick Continuous Silver Nanoshells. Langmuir 2020, 36, 4511–4518. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, J.; Wang, S.; Zhan, P.; Wang, Z.; Ming, N. Facile Methods to Coat Polystyrene and Silica Colloids with Metal. Adv. Funct. Mater. 2004, 14, 1089–1096. [Google Scholar] [CrossRef]
- Sobral-Filho, R.G.; Brito-Silva, A.M.; Isabelle, M.; Jirasek, A.; Lum, J.J.; Brolo, A.G. Plasmonic labeling of subcellular compartments in cancer cells: Multiplexing with fine-tuned gold and silver nanoshells. Chem. Sci. 2017, 8, 3038–3046. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, H.S.; Park, H.K. Facile Method To Prepare Surface-Enhanced-Raman-Scattering-Active Ag Nanostructures on Silica Spheres. Langmuir 2006, 22, 8083–8088. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-J.; Liu, C.-Y. Seed-Mediated Growth Technique for the Preparation of a Silver Nanoshell on a Silica Sphere. J. Phys. Chem. B 2003, 107, 12411–12415. [Google Scholar] [CrossRef]
- Tang, S.; Tang, Y.; Zhu, S.; Lu, H.; Meng, X. Synthesis and characterization of silica–silver core–shell composite particles with uniform thin silver layers. J. Solid State Chem. 2007, 180, 2871–2876. [Google Scholar] [CrossRef]
- Chen, D.; Liu, H.-Y.; Liu, J.-S.; Ren, X.-L.; Meng, X.-W.; Wu, W.; Tang, F.-Q. A general method for synthesis of continuous silver nanoshells on dielectric colloids. Thin Solid Films 2008, 516, 6371–6376. [Google Scholar] [CrossRef]
- Chateau, D.; Desert, A.; Lerouge, F.; Landaburu, G.; Santucci, S.; Parola, S. Beyond the Concentration Limitation in the Synthesis of Nanobipyramids and Other Pentatwinned Gold Nanostructures. ACS Appl. Mater. Interfaces 2019, 11, 39068–39076. [Google Scholar] [CrossRef] [PubMed]
- Le Beulze, A.; Duguet, E.; Mornet, S.; Majimel, J.; Tréguer-Delapierre, M.; Ravaine, S.; Florea, I.; Ersen, O. New Insights into the Side-Face Structure, Growth Aspects, and Reactivity of Agn Nanoprisms. Langmuir 2014, 30, 1424–1434. [Google Scholar] [CrossRef]
- Chen, M.; Kim, Y.N.; Lee, H.M.; Li, C.; Cho, S.O. Multifunctional Magnetic Silver Nanoshells with Sandwichlike Nanostructures. J. Phys. Chem. C 2008, 112, 8870–8874. [Google Scholar] [CrossRef]
- Yong, K.-T.; Sahoo, Y.; Swihart, M.T.; Prasad, P.N. Synthesis and plasmonic properties of silver and gold nanoshells on polystyrene cores of different size and of gold–silver core–shell nanostructures. Colloids Surfaces A Physicochem. Eng. Asp. 2006, 290, 89–105. [Google Scholar] [CrossRef]
- Kim, J.-H.; Bryan, W.W.; Lee, T.R. Preparation, Characterization, and Optical Properties of Gold, Silver, and Gold−Silver Alloy Nanoshells Having Silica Cores. Langmuir 2008, 24, 11147–11152. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, X.; Niu, C.; Wang, Y. Template-Activated Strategy toward One-Step Coating Silica Colloidal Microspheres with Sliver. ACS Appl. Mater. Interfaces 2013, 6, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.S.; Bouwman, J.; Chen, A.; Deutsch, M. Inorganic metallodielectric materials fabricated using two single-step methods based on the Tollen’s process. J. Colloid Interface Sci. 2007, 306, 41–49. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, Y.; Lakowicz, J.R. Luminescent Silica Core/Silver Shell Encapsulated with Eu(III) Complex. J. Phys. Chem. C 2009, 113, 19404–19410. [Google Scholar] [CrossRef] [PubMed]
- Bryan, W.W.; Jamison, A.C.; Chinwangso, P.; Rittikulsittichai, S.; Lee, T.-C. Preparation of THPC-generated silver, platinum, and palladium nanoparticles and their use in the synthesis of Ag, Pt, Pd, and Pt/Ag nanoshells. RSC Adv. 2016, 6, 68150–68159. [Google Scholar] [CrossRef]
- Purdy, S.C.; Muscat, A.J. Coating nonfunctionalized silica spheres with a high density of discrete silver nanoparticles. J. Nanoparticle Res. 2016, 18, 70. [Google Scholar] [CrossRef]
- Shen, Q.; Gao, H.; Xue, P.; Lü, Y.; Li, D.; Lian, L.; Liu, Y.; Liu, X. Surfactant-Free In Situ Synthesis of Sub-5 nm Silver Nanoparticles Embedded Silica Sub-Microspheres as Highly Efficient and Recyclable Catalysts. Chemistryselect 2018, 3, 10352–10356. [Google Scholar] [CrossRef]
- Pol, V.G.; Srivastava, D.N.; Palchik, O.; Slifkin, M.A.; Weiss, A.M.; Gedanken, A. Sonochemical Deposition of Silver Nanoparticles on Silica Spheres. Langmuir 2002, 18, 3352–3357. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Salgueiriño-Maceira, V.; Liz-Marzán, L.M. Deposition of Silver Nanoparticles on Silica Spheres by Pretreatment Steps in Electroless Plating. Chem. Mater. 2001, 13, 1630–1633. [Google Scholar] [CrossRef]
- Schierhorn, M.; Liz-Marzán, L.M. Synthesis of Bimetallic Colloids with Tailored Intermetallic Separation. Nano Lett. 2001, 2, 13–16. [Google Scholar] [CrossRef]
- Zhu, M.; Qian, G.; Hong, Z.; Wang, Z.; Fan, X.; Wang, M. Preparation and characterization of silica–silver core-shell structural submicrometer spheres. J. Phys. Chem. Solids 2005, 66, 748–752. [Google Scholar] [CrossRef]
- Hartlen, K.D.; Athanasopoulos, A.P.T.; Kitaev, V. Facile Preparation of Highly Monodisperse Small Silica Spheres (15 to >200 nm) Suitable for Colloidal Templating and Formation of Ordered Arrays. Langmuir 2008, 24, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.-B.; Fan, S.-G.; Zhao, C.-Y.; Wang, Q.-R.; Zhou, T.-Y.; Chen, X.; Yan, Z.-F.; Li, Y.-P.; Xing, W.; Wang, X.-D. A colorimetric agarose gel for formaldehyde measurement based on nanotechnology involving Tollens reaction. Chem. Commun. 2014, 50, 8121–8123. [Google Scholar] [CrossRef] [PubMed]
- Preston, T.C.; Signorell, R. Growth and Optical Properties of Gold Nanoshells Prior to the Formation of a Continuous Metallic Layer. ACS Nano 2009, 3, 3696–3706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, L.; Luo, J.; Zhao, X.; Wang, T.; Li, Y.; Fu, Y. Metallic Nanoshells with Sub-10 nm Thickness and Their Performance as Surface-Enhanced Spectroscopy Substrate. ACS Appl. Mater. Interfaces 2016, 8, 9889–9896. [Google Scholar] [CrossRef] [PubMed]
- Lermusiaux, L.; Plissonneau, M.; Bertry, L.; Drisko, G.L.; Buissette, V.; Le Mercier, T.; Duguet, E.; Tréguer-Delapierre, M. Seeded growth of ultrathin gold nanoshells using polymer additives and microwave radiation. Sci. Rep. 2021, 11, 17831. [Google Scholar] [CrossRef]
- Ma, Y.-W.; Zhang, J.; Zhang, L.-H.; Jian, G.-S.; Wu, S.-F. Theoretical Analysis the Optical Properties of Multi-coupled Silver Nanoshell Particles. Plasmonics 2011, 6, 705–713. [Google Scholar] [CrossRef]
- Norris, C.B.; Joseph, P.R.; Mackiewicz, M.R.; Reed, S.M. Minimizing Formaldehyde Use in the Synthesis of Gold−Silver Core−Shell Nanoparticles. Chem. Mater. 2010, 22, 3637–3645. [Google Scholar] [CrossRef]
- Plissoneau, M.; Bertry, L.; Le Mercier, T.; Buisette, V.; Duguet, E.; Drisko, G.L.; Tréguer-Delapierre, M.; Lermusiaux, L.; Leng, J.; Vynck, K.; et al. Nanoparticules Constituées d’un Cœur en Silice Recouvert de Cristallites en Argent. [Nanoparticles Consisting of a Silica Core Covered with Silver Crystallites]. World Patent WO/2022/048833, 10 March 2022. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2022048833 (accessed on 31 January 2023).








| ρ (μm−2) | T (°C) | SHGC (%) |
|---|---|---|
| 0 | 2.0 ± 0.5 | 100 |
| 3.3 | 5.9 ± 0.5 | 76 |
| 10 | 12.6 ± 0.5 | 58.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lermusiaux, L.; Roach, L.; Lehtihet, M.; Plissonneau, M.; Bertry, L.; Buissette, V.; Le Mercier, T.; Duguet, E.; Drisko, G.L.; Leng, J.; et al. Silver Nanoshells with Optimized Infrared Optical Response: Synthesis for Thin-Shell Formation, and Optical/Thermal Properties after Embedding in Polymeric Films. Nanomaterials 2023, 13, 614. https://doi.org/10.3390/nano13030614
Lermusiaux L, Roach L, Lehtihet M, Plissonneau M, Bertry L, Buissette V, Le Mercier T, Duguet E, Drisko GL, Leng J, et al. Silver Nanoshells with Optimized Infrared Optical Response: Synthesis for Thin-Shell Formation, and Optical/Thermal Properties after Embedding in Polymeric Films. Nanomaterials. 2023; 13(3):614. https://doi.org/10.3390/nano13030614
Chicago/Turabian StyleLermusiaux, Laurent, Lucien Roach, Moncef Lehtihet, Marie Plissonneau, Laure Bertry, Valérie Buissette, Thierry Le Mercier, Etienne Duguet, Glenna L. Drisko, Jacques Leng, and et al. 2023. "Silver Nanoshells with Optimized Infrared Optical Response: Synthesis for Thin-Shell Formation, and Optical/Thermal Properties after Embedding in Polymeric Films" Nanomaterials 13, no. 3: 614. https://doi.org/10.3390/nano13030614
APA StyleLermusiaux, L., Roach, L., Lehtihet, M., Plissonneau, M., Bertry, L., Buissette, V., Le Mercier, T., Duguet, E., Drisko, G. L., Leng, J., & Tréguer-Delapierre, M. (2023). Silver Nanoshells with Optimized Infrared Optical Response: Synthesis for Thin-Shell Formation, and Optical/Thermal Properties after Embedding in Polymeric Films. Nanomaterials, 13(3), 614. https://doi.org/10.3390/nano13030614