Grape Waste Materials—An Attractive Source for Developing Nanomaterials with Versatile Applications
Abstract
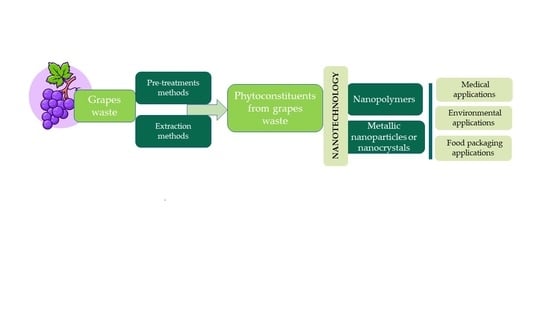
:1. Introduction
2. Recovery of Active Compounds from Grape Waste and Their Beneficial Effects
3. Potential Applications of Grape Wastes
3.1. Phenolic Compounds Obtained from Grape Wastes Used for Nanotechnological Formulations
3.1.1. Metallic Nanoparticles
3.1.2. Polymeric Nanocomposites
3.1.3. Lipidic Materials
3.2. Other Target Compounds from Grape Waste for Different Applications
4. Challenges and Perspectives for Grape by-Products
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sinha, S.; Tripathi, P. Trends and challenges in valorisation of food waste in developing economies: A case study of India. Case Stud. Chem. Environ. Eng. 2021, 4, 100162. [Google Scholar] [CrossRef]
- Theagarajan, R.; Malur Narayanaswamy, L.; Dutta, S.; Moses, J.A.; Chinnaswamy, A. Valorisation of grape pomace (cv. Muscat) for development of functional cookies. Int. J. Food Sci. Technol. 2019, 54, 1299–1305. [Google Scholar] [CrossRef]
- Kumar, V.; Sharma, N.; Umesh, M.; Selvaraj, M.; Al-Shehri, B.M.; Chakraborty, P.; Duhan, L.; Sharma, S.; Pasrija, R.; Awasthi, M.K.; et al. Emerging challenges for the agro-industrial food waste utilization: A review on food waste biorefinery. Bioresour. Technol. 2022, 362, 127790. [Google Scholar] [CrossRef]
- Mora-Garrido, A.B.; Cejudo-Bastante, M.J.; Heredia, F.J.; Escudero-Gilete, M.L. Revalorization of residues from the industrial exhaustion of grape by-products. LWT 2022, 156, 113057. [Google Scholar] [CrossRef]
- Scaffaro, R.; Citarrella, M.C.; Morreale, M. Green Composites Based on Mater-Bi® and Solanum lycopersicum Plant Waste for 3D Printing Applications. Polymers 2023, 15, 325. [Google Scholar] [CrossRef]
- Scaffaro, R.; Citarrella, M.C.; Gulino, E.F. Opuntia Ficus Indica based green composites for NPK fertilizer controlled release produced by compression molding and fused deposition modeling. Compos. Part A Appl. Sci. Manufacturing. 2022, 159, 107030. [Google Scholar] [CrossRef]
- Torres, F.G.; Rodriguez, S.; Saavedra, A.C. Green Composite Materials from Biopolymers Reinforced with Agroforestry Waste. J. Polym. Environ. 2019, 27, 2651–2673. [Google Scholar] [CrossRef]
- Scaffaro, R.; Citarrella, M.C.; Catania, A.; Settanni, L. Green composites based on biodegradable polymers and anchovy (Engraulis Encrasicolus) waste suitable for 3D printing applications. Compos. Sci. Technol. 2022, 230-1, 109768. [Google Scholar] [CrossRef]
- Syamimi, N.F.; Islam, M.R.; Sumdani, M.G.; Rashidi, N.M. Mechanical and thermal properties of snail shell particles-reinforced bisphenol-A bio-composites. Polym. Bull. 2022, 77, 2573–2589. [Google Scholar] [CrossRef]
- Hanny, A.; Islam, M.R.; Sumdani, M.G.; Rashidi, N.M. The effects of sintering on the properties of epoxy composites reinforced with chicken bone-based hydroxyapatites. Polym. Test. 2019, 78, 105987. [Google Scholar] [CrossRef]
- Wei, M.; Ma, T.; Ge, Q.; Li, C.; Zhang, K.; Fang, Y.; Sun, X. Challenges and opportunities of winter vine pruning for global grape and wine industries. J. Clean. Prod. 2022, 380, 135086. [Google Scholar] [CrossRef]
- IOVW (International Organization of Vineyards and Wine) Statistics. Available online: https://www.oiv.int/en/statistiques/recherche (accessed on 12 December 2022).
- Ilyas, T.; Chowdhary, P.; Chaurasia, D.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Sustainable green processing of grape pomace for the production of value-added products: An overview. Environ. Technol. Innov. 2021, 23, 101592. [Google Scholar] [CrossRef]
- Jesus, M.S.; Romaní, A.; Genisheva, Z.; Teixeira, J.A.; Domingues, L. Integral valorization of vine pruning residue by sequential autohydrolysis stages. J. Clean. Prod. 2017, 168, 74–86. [Google Scholar] [CrossRef] [Green Version]
- Huynh, K.H.; Lee, K.Y.; Chang, H.; Lee, S.H.; Kim, J.; Pham, X.H.; Lee, Y.S.; Rho, W.Y.; Jun, B.H. Bioapplications of Nanomaterials. Adv. Exp. Med. Biol. 2021, 1309, 235–255. [Google Scholar] [CrossRef]
- Ferrara, A.; Ferranti, P. Technological processes to produce novel ingredients from agri-food sources: Functional compounds from enological wastes. In Reference Module in Food Science; Elsevier: Amsterdam, Netherlands, 2023. [Google Scholar]
- Duque-Acevedo, M.; Belmonte-Ureña, L.J.; Cortés-García, F.J.; Camacho-Ferre, F. Agricultural waste: Review of the evolution, approaches and perspectives on alternative uses. Glob. Ecol. Conserv. 2020, 22, e00902. [Google Scholar] [CrossRef]
- Senthilkumar, K.; Naveen Kumar, M.; Chitra Devi, V.; Saravanan, K.; Easwaramoorthi, S. Agro-industrial waste valorization to energy and value added products for environmental sustainability. In Biomass Valorization to Bioenergy; Praveen Kumar, R., Bharathiraja, B., Kataki, R., Moholkar, V.S., Eds.; Springer: Singapore, 2020; pp. 1–9. [Google Scholar]
- Pereira, R.N.; Coelho, M.; Genisheva, Z.; Fernandes, J.-M.; Vicente, A.A.; Pintado, M.E.; Teixeira, J.A. Using Ohmic Heating effect on grape skins as a pretreatment for anthocyanins extraction. Food Bioprod. Process. 2020, 124, 320–328. [Google Scholar] [CrossRef]
- Ul Haq, I.; Qaisar, K.; Nawaz, A.; Akram, F.; Mukhtar, H.; Zohu, X.; Xu, Y.; Mumtaz, M.W.; Rashid, U.; Ghani, W.A.W.A.K.; et al. Advances in Valorization of Lignocellulosic Biomass towards Energy Generation. Catalysts 2021, 11, 309. [Google Scholar] [CrossRef]
- Dey, T.; Bhattacharjee, T.; Nag, P.; Ritika; Ghati, A.; Kuila, A. Valorization of agro-waste into value added products for sustainable development. Bioresour. Technol. Rep. 2021, 16, 100834. [Google Scholar] [CrossRef]
- Ning, P.; Yang, G.; Hu, L.; Sun, J.; Shi, L.; Zhou, Y.; Wang, Z.; Yang, J. Recent advances in the valorization of plant biomass. Biotechnol. Biofuels 2021, 14, 102. [Google Scholar] [CrossRef]
- Philippini, R.R.; Martiniano, S.E.; Ingle, A.P.; Franco Marcelino, P.R.; Silva, G.M.; Barbosa, F.G.; dos Santos, J.C.; da Silva, S.S. Agroindustrial Byproducts for the Generation of Biobased Products: Alternatives for Sustainable Biorefineries. Front. Energy Res. 2020, 8, 152. [Google Scholar] [CrossRef]
- Mora-Sandí, A.; Ramírez-González, A.; Castillo-Henríquez, L.; Lopretti-Correa, M.; Vega-Baudrit, J.R. Persea Americana Agro-Industrial Waste Biorefinery for Sustainable High-Value-Added Products. Polymers 2021, 13, 1727. [Google Scholar] [CrossRef]
- IOVW (International Organization of Vineyards and Wine). Available online: https://www.oiv.int/public/medias/6782/oiv-2019-statistical-report-on-world-vitiviniculture.pdf (accessed on 12 December 2022).
- Keller, M. Chapter 1—Taxonomy and anatomy. In The Science of Grapevines, 3rd ed.; Keller, M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–60. [Google Scholar]
- Schoedl, K.; Schuhmacher, R.; Forneck, A. Studying the polyphenols of grapevine leaves according to age and insertion level under controlled conditions. Sci. Hortic. 2012, 141, 37–41. [Google Scholar] [CrossRef]
- Goufo, P.; Singh, R.K.; Cortez, I. A Reference List of Phenolic Compounds (Including Stilbenes) in Grapevine (Vitis vinifera L.) Roots, Woods, Canes, Stems, and Leaves. Antioxidants 2020, 9, 398. [Google Scholar] [CrossRef] [PubMed]
- Max, B.; Salgado, J.M.; Cortés, S.; Domínguez, J.M. Extraction of Phenolic Acids by Alkaline Hydrolysis from the Solid Residue Obtained after Prehydrolysis of Trimming Vine Shoots. J. Agric. Food Chem. 2010, 58, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Gabaston, J.; Cantos-Villar, E.; Biais, B.; Waffo-Teguo, P.; Renouf, E.; Corio-Costet, M.F.; Richard, T.; Mérillon, J.M. Stilbenes from Vitis vinifera L. Waste: A Sustainable Tool for Controlling Plasmopara Viticola. J. Agric. Food Chem. 2017, 65, 2711–2718. [Google Scholar] [CrossRef]
- Contreras, M.d.M.; Romero-García, J.M.; López-Linares, J.C.; Romero, I.; Castro, E. Residues from grapevine and wine production as feedstock for a biorefinery. Food Bioprod. Process. 2022, 134, 56–79. [Google Scholar] [CrossRef]
- Jin, Q.; Neilson, A.P.; Stewart, A.C.; O’Keefe, S.F.; Kim, Y.-T.; McGuire, M.; Wilder, G.; Huang, H. Integrated Approach for the Valorization of Red Grape Pomace: Production of Oil, Polyphenols, and Acetone–Butanol–Ethanol. ACS Sustain. Chem. Eng. 2018, 6, 16279–16286. [Google Scholar] [CrossRef]
- Ioannidou, S.P.; Margellou, A.G.; Petala, M.D.; Triantafyllidis, K.S. Pretreatment/fractionation and characterization of winery waste streams within an integrated biorefinery concept. Sustain. Chem. Pharm. 2022, 27, 100670. [Google Scholar] [CrossRef]
- ISTAT (Istituto Nazionale di Istatistica). Available online: http://dati.istat.it/Index.aspx?QueryId=33706 (accessed on 12 December 2022).
- Perra, M.; Bacchetta, G.; Muntoni, A.; De Gioannis, G.; Castangia, I.; Rajha, H.N.; Manca, M.L.; Manconi, M. An outlook on modern and sustainable approaches to the management of grape pomace by integrating green processes, biotechnologies and advanced biomedical approaches. J. Funct. Foods 2022, 98, 105276. [Google Scholar] [CrossRef]
- Kandylis, P.; Dimitrellou, D.; Moschakis, T. Recent applications of grapes and their derivatives in dairy products. Trends Food Sci. Technol. 2021, 114, 696–711. [Google Scholar] [CrossRef]
- Bender, A.B.B.; Speroni, C.S.; Moro, K.I.B.; Morisso, F.D.P.; dos Santos, D.R.; da Silva, L.P.; Penna, N.G. Effects of micronization on dietary fiber composition, physicochemical properties, phenolic compounds, and antioxidant capacity of grape pomace and its dietary fiber concentrate. LWT 2020, 117, 108652. [Google Scholar] [CrossRef]
- Benbouguerra, N.; Richard, T.; Saucier, C.; Garcia, F. Voltammetric Behavior, Flavanol and Anthocyanin Contents, and Antioxidant Capacity of Grape Skins and Seeds during Ripening (Vitis vinifera var. Merlot, Tannat, and Syrah). Antioxidants 2020, 9, 800. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Du, C. Could grape-based food supplements prevent the development of chronic kidney disease? Crit. Rev. Food Sci. Nutr. 2020, 60, 3054–3062. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Simon, J.E.; Wu, Q. A critical review on grape polyphenols for neuroprotection: Strategies to enhance bioefficacy. Crit. Rev. Food Sci. Nutr. 2020, 60, 597–625. [Google Scholar] [CrossRef] [PubMed]
- Mohamedshah, Z.; Chadwick-Corbin, S.; Wightman, J.D.; Ferruzzi, M.G. Comparative assessment of phenolic bioaccessibility from 100% grape juice and whole grapes. Food Funct. 2020, 11, 6433–6445. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Bae, H. An Overview of Stress-Induced Resveratrol Synthesis in Grapes: Perspectives for Resveratrol-Enriched Grape Products. Molecules 2017, 22, 294. [Google Scholar] [CrossRef] [Green Version]
- Calabriso, N.; Massaro, M.; Scoditti, E.; Verri, T.; Barca, A.; Gerardi, C.; Giovinazzo, G.; Carluccio, M.A. Grape Pomace Extract Attenuates Inflammatory Response in Intestinal Epithelial and Endothelial Cells: Potential Health-Promoting Properties in Bowel Inflammation. Nutrients 2022, 14, 1175. [Google Scholar] [CrossRef]
- Leal, C.; Gouvinhas, I.; Santos, R.A.; Rosa, E.; Silva, A.M.; Saavedra, M.J.; Barros, A.I.R.N.A. Potential application of grape (Vitis vinifera L.) stem extracts in the cosmetic and pharmaceutical industries: Valorization of a by-product. Ind. Crops Prod. 2020, 154, 112675. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Di Lorenzo, C.; Colombo, E.; Colombo, F.; Fumagalli, M.; Frigerio, G.; Restani, P.; Dell’Agli, M. The effect of in vitro gastrointestinal digestion on the anti-inflammatory activity of Vitis vinifera L. leaves. Food Funct. 2015, 6, 2453–2463. [Google Scholar] [CrossRef]
- Chakraborty, D.; Chatterjee, S.; Althuri, A.; Ganesh Palani, S.; Venkata Mohan, S. Sustainable enzymatic treatment of organic waste in a framework of circular economy. Bioresour. Technol. 2023, 370, 128487. [Google Scholar] [CrossRef]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape Pomace as a Sustainable Source of Bioactive Compounds: Extraction, Characterization, and Biotechnological Applications of Phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef] [PubMed]
- Fierascu, R.C.; Fierascu, I.; Avramescu, S.M.; Sieniawska, E. Recovery of Natural Antioxidants from Agro-Industrial Side Streams through Advanced Extraction Techniques. Molecules 2019, 24, 4212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatke, P.; Rajan, M. Comparison of Conventional and Novel Extraction Techniques for the Extraction of Scopoletin from Convolvulus Pluricaulis. Indian J. Pharm. Educ. Res. 2014, 48, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D.; et al. Current applications and new opportunities for the use of pulsed electric fields in food science and industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Vergara-Salinas, J.R.; Bulnes, P.; Zúñiga, M.C.; Pérez-Jiménez, J.; Torres, J.L.; Mateos-Martín, M.L.; Agosin, E.; Pérez-Correa, J.R. Effect of pressurized hot water extraction on antioxidants from grape pomace before and after enological fermentation. J. Agric. Food Chem. 2013, 61, 6929–6936. [Google Scholar] [CrossRef]
- Donsì, F.; Ferrari, G.; Fruilo, M.; Pataro, G. Pulsed electric field-assisted vinification of aglianico and piedirosso grapes. J. Agric. Food Chem. 2010, 58, 11606–11615. [Google Scholar] [CrossRef]
- Boussetta, N.; Vorobiev, E.; Le, L.H.; Cordin-Falcimaigne, A.; Lanoisellé, J.L. Application of electrical treatments in alcoholic solvent for polyphenols extraction from grape seeds. LWT—Food Sci. Technol. 2012, 46, 127–134. [Google Scholar] [CrossRef]
- Ntourtoglou, G.; Drosou, F.; Chatzimitakos, T.; Athanasiadis, V.; Bozinou, E.; Dourtoglou, V.G.; Elhakem, A.; Sami, R.; Ashour, A.A.; Shafie, A.; et al. Combination of Pulsed Electric Field and Ultrasound in the Extraction of Polyphenols and Volatile Compounds from Grape Stems. Appl. Sci. 2022, 12, 6219. [Google Scholar] [CrossRef]
- Corrales, M.; García, A.F.; Butz, P.; Tauscher, B. Extraction of anthocyanins from grape skins assisted by high hydrostatic pressure. J. Food Eng. 2009, 90, 415–421. [Google Scholar] [CrossRef]
- Sólyom, K.; Solá, R.; Cocero, M.J.; Mato, R.B. Thermal degradation of grape marc polyphenols. Food Chem. 2014, 159, 361–366. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Rosselló, C.; Simal, S.; Garau, M.C.; López, F.; Femenia, A. Physico-chemical properties of cell wall materials obtained from ten grape varieties and their byproducts: Grape pomaces and stems. LWT—Food Sci. Technol. 2010, 43, 1580–1586. [Google Scholar] [CrossRef]
- Devesa-Rey, R.; Vecino, X.; Varela-Alende, J.L.; Barral, M.T.; Cruz, J.M.; Moldes, A.B. Valorization of winery waste vs. the costs of not recycling. Waste Manag. 2011, 31, 2327–2335. [Google Scholar] [CrossRef]
- Beres, C.; Simas-Tosin, F.F.; Cabezudo, I.; Freitas, S.P.; Iacomini, M.; Mellinger-Silva, C.; Cabral, L.M.C. Antioxidant dietary fibre recovery from Brazilian Pinot noir grape pomace. Food Chem. 2016, 201, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Karovičová, J.; Kohajdová, Z.; Minarovičová, L.; Kuchtová, V. The Chemical Composition of Grape Fibre. Potravin. Slovak J. Food Sci. 2015, 9, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Coelho, M.C.; Pereira, R.N.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. The use of emergent technologies to extract added value compounds from grape by-products. Trends Food Sci. Technol. 2020, 106, 182–197. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Zheng, L.; Li, J. Advance on the bioactivity and potential applications of dietary fibre from grape pomace. Food Chem. 2015, 186, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Wang, Y.; Sun, Z.; Cui, C.; Li, H.; Luo, K.; Cheng, A. Effects of steam explosion on phenolic compounds and dietary fiber of grape pomace. LWT 2023, 173, 114350. [Google Scholar] [CrossRef]
- Sheng, K.; Qu, H.; Liu, C.; Yan, L.; You, J.; Shui, S.; Zheng, L. A comparative assess of high hydrostatic pressure and superfine grinding on physicochemical and antioxidant properties of grape pomace. Int. J. Food Sci. Technol. 2017, 52, 2106–2114. [Google Scholar] [CrossRef]
- Chamorro, S.; Viveros, A.; Alvarez, I.; Vega, E.; Brenes, A. Changes in polyphenol and polysaccharide content of grape seed extract and grape pomace after enzymatic treatment. Food Chem. 2012, 133, 308–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Górnaś, P.; Rudzińska, M.; Grygier, A.; Lācis, G. Diversity of oil yield, fatty acids, tocopherols, tocotrienols, and sterols in the seeds of 19 interspecific grapes crosses. J. Sci. Food Agric. 2019, 99, 2078–2087. [Google Scholar] [CrossRef]
- Ovcharova, T.; Zlatanov, M.; Dimitrova, R. Chemical composition of seeds of four Bulgarian grape varieties. Ciência Téc. Vitiv. 2016, 31, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Wen, X.; Zhu, M.; Hu, R.; Zhao, J.; Chen, Z.; Li, J.; Ni, Y. Characterisation of seed oils from different grape cultivars grown in China. J. Food Sci. Technol. 2016, 53, 3129–3136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rombaut, N.; Savoire, R.; Thomasset, B.; Castello, J.; Van Hecke, E.; Lanoisellé, J.-L. Optimization of oil yield and oil total phenolic content during grape seed cold screw pressing. Ind. Crops Prod. 2015, 63, 26–33. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Cruz, R.; Pereira, J.A.; Ramalhosa, E. Seed oils of ten traditional Portuguese grape varieties with interesting chemical and antioxidant properties. Food Res. Int. 2013, 50, 161–166. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Wei, J.; Xue, Y.; Lan, S.; Li, X.; Yu, D.; Wang, J. Extracting oil from grape seed using a combined wet enzymatic process and pressing. Innov. Food Sci. Emerg. Technol. 2022, 77, 102941. [Google Scholar] [CrossRef]
- Ealia, A.M.; Saravanakumar, M.P. A review on the classification, characterisation, synthesis of nanoparticles and their application. IOP Conf. Ser. Mater. Sci. Eng. 2017, 263, 032019. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications—An updated report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef]
- Ramteke, C.; Chakrabarti, T.; Sarangi, B.K.; Pandey, R.-A. Synthesis of Silver Nanoparticles from the Aqueous Extract of Leaves of <i>Ocimum sanctum</i> for Enhanced Antibacterial Activity. J. Chem. 2013, 2013, 278925. [Google Scholar]
- Simonetti, G.; Palocci, C.; Valletta, A.; Kolesova, O.; Chronopoulou, L.; Donati, L.; Di Nitto, A.; Brasili, E.; Tomai, P.; Gentili, A.; et al. Anti-Candida Biofilm Activity of Pterostilbene or Crude Extract from Non-Fermented Grape Pomace Entrapped in Biopolymeric Nanoparticles. Molecules 2019, 24, 2070. [Google Scholar] [CrossRef] [Green Version]
- Patra, J.K.; Baek, K.H. Novel green synthesis of gold nanoparticles using Citrullus lanatus rind and investigation of proteasome inhibitory activity, antibacterial, and antioxidant potential. Int. J. Nanomed. 2015, 10, 7253–7264. [Google Scholar]
- Tade, R.S.; Nangare, S.N.; Patil, P.O. Agro-Industrial Waste-Mediated Green Synthesis of Silver Nanoparticles and Evaluation of Its Antibacterial Activity. Nano Biomed. Eng. 2020, 12, 57–66. [Google Scholar] [CrossRef]
- Sutan, N.A.; Manolescu, D.S.; Fierascu, I.; Neblea, A.M.; Sutan, C.; Ducu, C.; Soare, L.C.; Negrea, D.; Avramescu, S.M.; Fierascu, R.C. Phytosynthesis of gold and silver nanoparticles enhance in vitro antioxidant and mitostimulatory activity of Aconitum toxicum Reichenb. rhizomes alcoholic extracts. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 746–758. [Google Scholar] [CrossRef]
- Fierascu, I.; Georgiev, M.I.; Ortan, A.; Fierascu, R.C.; Avramescu, S.M.; Ionescu, D.; Sutan, A.; Brinzan, A.; Ditu, L.M. Phyto-mediated metallic nanoarchitectures via Melissa ofcinalis L.: Synthesis, characterization and biological properties. Sci. Rep. 2017, 7, 12428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Tian, R.; Zhou, J.; Liu, Y. Multifunctional chitosan/grape seed extract/silver nanoparticle composite for food packaging application. Int. J. Biol. Macromol. 2022, 207, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Soto, K.M.; Quezada-Cervantes, C.T.; Hernández-Iturriaga, M.; Luna-Bárcenas, G.; Vazquez-Duhalt, R.; Mendoza, S. Fruit peels waste for the green synthesis of silver nanoparticles with antimicrobial activity against foodborne pathogens. LWT 2019, 103, 293–300. [Google Scholar] [CrossRef]
- Montagner, G.E.; Wingert, N.R.; Stein, C.d.S.; Moresco, R.N.; Fogaça, A.d.O.; Gomes, P. Optimization of the extraction of antioxidant compounds from grape seed from winemaking waste. Sustain. Chem. Pharm. 2022, 30, 100856. [Google Scholar] [CrossRef]
- Gubitosa, J.; Rizzi, V.; Laurenzana, A.; Scavone, F.; Frediani, E.; Fibbi, G.; Fanelli, F.; Sibillano, T.; Giannini, C.; Fini, P.; et al. The “End Life” of the Grape Pomace Waste Become the New Beginning: The Development of a Virtuous Cycle for the Green Synthesis of Gold Nanoparticles and Removal of Emerging Contaminants from Water. Antioxidants 2022, 11, 994. [Google Scholar] [CrossRef]
- Saratale, G.D.; Saratale, R.G.; Kim, D.-S.; Kim, D.-Y.; Shin, H.-S. Exploiting Fruit Waste Grape Pomace for Silver Nanoparticles Synthesis, Assessing Their Antioxidant, Antidiabetic Potential and Antibacterial Activity Against Human Pathogens: A Novel Approach. Nanomaterials 2020, 10, 1457. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Ahn, S.; Shin, H.S. Grape Pomace Extracted Tannin for Green Synthesis of Silver Nanoparticles: Assessment of Their Antidiabetic, Antioxidant Potential and Antimicrobial Activity. Polymers 2021, 13, 4355. [Google Scholar] [CrossRef]
- Ping, Y.; Zhang, J.; Xing, T.; Chen, G.; Tao, R.; Choo, K.-H. Green synthesis of silver nanoparticles using grape seed extract and their application for reductive catalysis of Direct Orange 26. J. Ind. Eng. Chem. 2018, 58, 74–79. [Google Scholar] [CrossRef]
- Raota, C.S.; Cerbaro, A.F.; Salvador, M.; Delamare, A.P.L.; Echeverrigaray, S.; da Silva Crespo, J.; da Silva, T.B.; Giovanela, M. Green synthesis of silver nanoparticles using an extract of Ives cultivar (Vitis labrusca) pomace: Characterization and application in wastewater disinfection. J. Environ. Chem. Eng. 2019, 7, 103383. [Google Scholar] [CrossRef]
- Nivetha, K.; Vinotha, V.; Albeshr, M.F.; Mahboob, S.; Manzoor, I.; Govindarajan, M.; Vaseeharan, B. Synthesis and characterization of Vitis vinifera exocarp-mediated ZnO nanoparticles: An evaluation of biological potential and ecotoxicity. J. Drug Deliv. Sci. Technol. 2022, 77, 103846. [Google Scholar] [CrossRef]
- Wu, Z.; Deng, W.; Luo, J.; Deng, D. Multifunctional nano-cellulose composite films with grape seed extracts and immobilized silver nanoparticles. Carbohydr. Polym. 2019, 205, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.R.; Xavier, M.; Amado, I.R.; Gonçalves, C.; Castro, P.M.; Tonon, R.V.; Cabral, L.M.C.; Pastrana, L.; Pintado, M.E. Polymeric nanoparticles as oral delivery systems for a grape pomace extract towards the improvement of biological activities. Mater. Sci. Eng. C 2021, 119, 111551. [Google Scholar] [CrossRef]
- Khan, A.; Khan, R.A.; Salmieri, S.; Le Tien, C.; Riedl, B.; Bouchard, J.; Chauve, G.; Tan, V.; Kamal, M.R.; Lacroix, M. Mechanical and barrier properties of nanocrystalline cellulose reinforced chitosan based nanocomposite films. Carbohydr. Polym. 2012, 90, 1601–1608. [Google Scholar] [CrossRef]
- Bangar, S.P.; Whiteside, W.S. Nano-cellulose reinforced starch bio composite films- A review on green composites. Int. J. Biol. Macromol. 2021, 185, 849–860. [Google Scholar] [CrossRef]
- Xu, Y.; Rehmani, N.; Alsubaie, L.; Kim, C.; Sismour, E.; Scales, A. Tapioca starch active nanocomposite films and their antimicrobial effectiveness on ready-to-eat chicken meat. Food Packag. Shelf Life 2018, 16, 86–91. [Google Scholar] [CrossRef]
- Perra, M.; Manca, M.L.; Tuberoso, C.I.G.; Caddeo, C.; Marongiu, F.; Peris, J.E.; Orrù, G.; Ibba, A.; Fernàndez-Busquets, X.; Fattouch, S.; et al. A green and cost-effective approach for the efficient conversion of grape byproducts into innovative delivery systems tailored to ensure intestinal protection and gut microbiota fortification. Innov. Food Sci. Emerg. Technol. 2022, 80, 103103. [Google Scholar] [CrossRef]
- Manconi, M.; Marongiu, F.; Castangia, I.; Manca, M.L.; Caddeo, C.; Tuberoso, C.I.; D’Hallewin, G.; Bacchetta, G.; Fadda, A.M. Polymer-associated liposomes for the oral delivery of grape pomace extract. Colloids Surf. B Biointerfaces 2016, 146, 910–917. [Google Scholar] [CrossRef]
- Manca, M.L.; Marongiu, F.; Castangia, I.; Catalán-Latorre, A.; Caddeo, C.; Bacchetta, G.; Ennas, G.; Zaru, M.; Fadda, A.M.; Manconi, M. Protective effect of grape extract phospholipid vesicles against oxidative stress skin damages. Ind. Crops Prod. 2016, 83, 561–567. [Google Scholar] [CrossRef]
- Gibis, M.; Thellmann, K.; Thongkaew, C.; Weiss, J. Interaction of polyphenols and multilayered liposomal-encapsulated grape seed extract with native and heat-treated proteins. Food Hydrocoll. 2014, 41, 119–131. [Google Scholar] [CrossRef]
- Manca, M.L.; Casula, E.; Marongiu, F.; Bacchetta, G.; Sarais, G.; Zaru, M.; Escribano-Ferrer, E.; Peris, J.E.; Usach, I.; Fais, S.; et al. From waste to health: Sustainable exploitation of grape pomace seed extract to manufacture antioxidant, regenerative and prebiotic nanovesicles within circular economy. Sci. Rep. 2020, 10, 14184. [Google Scholar] [CrossRef] [PubMed]
- García-Lomillo, J.; González-SanJosé, M.L.; Del Pino-García, R.; Rivero-Pérez, M.D.; Muñiz-Rodríguez, P. Antioxidant and antimicrobial properties of wine byproducts and their potential uses in the food industry. J. Agric. Food Chem. 2014, 62, 12595–12602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pazos, M.; Gallardo, J.M.; Torres, J.L.; Medina, I. Activity of grape polyphenols as inhibitors of the oxidation of fish lipids and frozen fish muscle. Food Chem. 2005, 92, 547–557. [Google Scholar] [CrossRef]
- Garrido, M.D.; Auqui, M.; Martí, N.; Linares, M.B. Effect of two different red grape pomace extracts obtained under different extraction systems on meat quality of pork burgers. LWT—Food Sci. Technol. 2011, 44, 2238–2243. [Google Scholar] [CrossRef]
- Guerra-Rivas, C.; Vieira, C.; Rubio, B.; Martínez, B.; Gallardo, B.; Mantecón, A.R.; Lavín, P.; Manso, T. Effects of grape pomace in growing lamb diets compared with vitamin E and grape seed extract on meat shelf life. Meat Sci. 2016, 116, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Šporin, M.; Avbelj, M.; Kovač, B.; Možina, S.S. Quality characteristics of wheat flour dough and bread containing grape pomace flour. Food Sci. Technol. Int. 2018, 24, 251–263. [Google Scholar] [CrossRef]
- Karnopp, A.R.; Oliveira, K.G.; de Andrade, E.F.; Postingher, B.M.; Granato, D. Optimization of an organic yogurt based on sensorial, nutritional, and functional perspectives. Food Chem. 2017, 233, 401–411. [Google Scholar] [CrossRef]
- Kalli, E.; Lappa, I.; Bouchagier, P.; Tarantilis, P.A.; Skotti, E. Novel application and industrial exploitation of winery by-products. Bioresour. Bioprocess. 2018, 5, 46. [Google Scholar] [CrossRef]
- Sánchez-Alonso, I.; Jiménez-Escrig, A.; Saura-Calixto, F.; Borderías, A.J. Effect of grape antioxidant dietary fibre on the prevention of lipid oxidation in minced fish: Evaluation by different methodologies. Food Chem. 2007, 101, 372–378. [Google Scholar] [CrossRef]
- Caldas, T.W.; Mazza, K.E.L.; Teles, A.S.C.; Mattos, G.N.; Brígida, A.I.S.; Conte-Junior, C.A.; Borguini, R.G.; Godoy, R.L.O.; Cabral, L.M.C.; Tonon, R.V. Phenolic compounds recovery from grape skin using conventional and non-conventional extraction methods. Ind. Crops Prod. 2018, 111, 86–91. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B. An overview of the recent trends on the waste valorization techniques for food wastes. J. Environ. Manag. 2019, 233, 352–370. [Google Scholar] [CrossRef] [PubMed]
- Samaddar, P.; Sik Ok, Y.; Kim, K.H.; Kwon, E.E.; Tsang, D.C.W. Synthesis of nanomaterials from various wastes and their new age applications. J. Clean. Prod. 2018, 197, 1190–1209. [Google Scholar] [CrossRef]






| Pretreatment | Advantages | Disadvantages | Matrix for Which the Pretreatment Is Applicable | Reference |
|---|---|---|---|---|
| Hydrothermal | Does not involve chemical reagents | Decreased yield of lignin recovery | Materials with a poor lignin content | [20] |
| Irradiation | Depolymerization of cellulose and solubilization of lignin. | High costs | Woody materials with a high content of lignin | [21] |
| Alkaline pre-treatment | Solubilization of different compounds with high yields | Large amounts of water required for removing chemical compounds Large amounts of reagents required | Wastes containing lignin | [22] |
| Supercritical CO2 | Low temperatures suitable for degradable compounds | Expensive process; special conditions required | Material with high lignin content | [23] |
| Biological method | Environment-friendly, low consumption of energy | Process is mild, with a slow rate of hydrolysis | Material with high lignin, cellulose, and polysaccharides content | [24] |
| Nanomaterial | Plant Waste/Type of Extract | Extraction Method | Nanomaterial Properties | Application | Reference |
|---|---|---|---|---|---|
| Food packaging application | |||||
| AgNP | GSE/fresh juice | - | spherical; 23.8 nm and 12.1 nm zeta potential—−37.9 mV crystallite size—9.9 nm | Antimicrobial effect against A. niger ATCC16404 and P. chrysogenum T16 Materials were used as coatings to maintain the postharvest quality of grapes | [80] |
| GPE/classical temperature extraction | 100 mL of deionized water and heated at 60 °C for 10 min | spherical; 3 to 14 nm | Antimicrobial effect against Escherichia coli O157:H7 and Listeria monocytogenes | [81] | |
| Environmental protection | |||||
| AgNP | GSE/fresh juice | - | average size of the AgNPs—54.8 nm | Reductive degradation of Direct Orange 26 in the presence of NaBH4 | [86] |
| Vitis labrusca pomace | 150 mL of a hydroalcoholic solution (50% v/v); the mixture was centrifuged for 5 min at 5000 rpm and filtered | spherical and polyhedral shapes; 2.9–55 nm | Inhibition—75.3% for Staphylococcus aureus and 15.2% for Enterococcus faecalis in wastewater | [87] | |
| Medical application | |||||
| ZnO NPs | Grape peels/aqueous extract | water extract (1:10), 50 °C, 1 h | nanocones, average size—19.36 nm | Bactericidal activity against S. aureus and P. aeruginosa; MIC—40 μg/mL; zone of inhibition (mm)—2–6 | [88] |
| AuNP | Grape pomace/aqueous extract | 2 g of grape waste in 50 mL of deionized water, boiled for 5 min | 30 nm wide, having a cubic Au phase | antioxidants and tyrosinase inhibitors; sun protection cream | [83] |
| AgNP | Grape pomace/ethanolic extract | solid phase: ethanol—1:20 | 20–35 nm | Antioxidant and antibacterial properties (Escherichia coli and Staphylococcus aureus) | [84] |
| AgNP | Grape pomace/aqueous extract | aqueous-based solution at 80 °C for 4 h | face centered cubic (FCC) crystal structure; 15 to 20 nm | Antidiabetic, antioxidant potential, and antimicrobial activity | [85] |
| Nanocellulose/grape seed extract/AgNPs | Grape seed extracts -commercial | - | charred residue of TNC/GSE/AgNPs film—33.49% optical transmittance of 80–93% oxygen permeability (OP)—1.027 cm3 m−2·24 h−1·0.1 MPa−1 | E. coli—zone of inhibition (mm)—2 S. aureus—zone of inhibition (mm)—6 | [89] |
| Chitosan/alginate nanoparticles | Grape pomace extract | enzymatic extraction followed by freeze-drying (xylanase produced by Aspergillus niger 3T5B8 and Viscozyme® enzymatic commercial cocktail—(from Novozymes Bagsvaerd, Denmark) | zeta potential (mV): −15 to −25.2 nanoparticle size—400 and 1000 nm | 2-log reduction of L. monocytogenes, P. aeruginosa and S. enteritidis, and a 1-log reduction of E. coli | [90] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baroi, A.M.; Sieniawska, E.; Świątek, Ł.; Fierascu, I. Grape Waste Materials—An Attractive Source for Developing Nanomaterials with Versatile Applications. Nanomaterials 2023, 13, 836. https://doi.org/10.3390/nano13050836
Baroi AM, Sieniawska E, Świątek Ł, Fierascu I. Grape Waste Materials—An Attractive Source for Developing Nanomaterials with Versatile Applications. Nanomaterials. 2023; 13(5):836. https://doi.org/10.3390/nano13050836
Chicago/Turabian StyleBaroi, Anda Maria, Elwira Sieniawska, Łukasz Świątek, and Irina Fierascu. 2023. "Grape Waste Materials—An Attractive Source for Developing Nanomaterials with Versatile Applications" Nanomaterials 13, no. 5: 836. https://doi.org/10.3390/nano13050836
APA StyleBaroi, A. M., Sieniawska, E., Świątek, Ł., & Fierascu, I. (2023). Grape Waste Materials—An Attractive Source for Developing Nanomaterials with Versatile Applications. Nanomaterials, 13(5), 836. https://doi.org/10.3390/nano13050836