Facile One-Pot Green Synthesis of Magneto-Luminescent Bimetallic Nanocomposites with Potential as Dual Imaging Agent
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals for Syntheses
2.2. Chemicals for Alamar Blue Assay
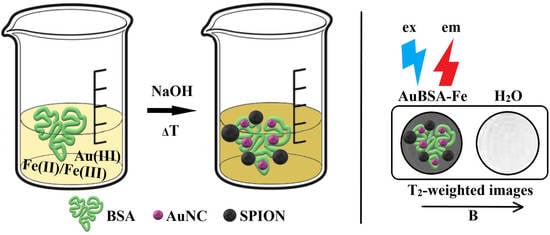
2.3. Syntheses of AuBSA and AuBSA-Fe—Their Purification, Concentrate Formation, and Storage
2.4. Characterization Techniques
2.4.1. Fluorescence Spectroscopy
2.4.2. HR-TEM, STEM, and EDS
2.4.3. Mössbauer Spectroscopy
2.4.4. XPS
2.4.5. ICP-MS
2.4.6. MR Relaxometry and Imaging
2.4.7. Alamar Blue Assay (Resazurin Assay)
3. Results and Discussion
3.1. Luminescent Properties of AuBSA-Fe in Comparison to AuBSA
3.2. Investigation of Morphology and Particle Size Distribution in Luminescent AuBSA-Fe
3.3. Evidence of SPIONs in Luminescent AuBSA-Fe via Mössbauer Spectroscopy
3.4. Application of Luminescent AuBSA-Fe as MRI Contrast Agents
3.5. Stability and Biocompatibility of AuBSA-Fe Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, X.; Xu, K.; Taratula, O.; Farsad, K. Applications of Nanoparticles in Biomedical Imaging. Nanoscale 2019, 11, 799–819. [Google Scholar] [CrossRef]
- Nienhaus, K.; Wang, H.; Nienhaus, G.U. Nanoparticles for Biomedical Applications: Exploring and Exploiting Molecular Interactions at the Nano-Bio Interface. Mater. Today Adv. 2020, 5, 100036. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Y.; Zhang, C.; Jia, Y.; Luo, Y.; Gao, X. AuGd Integrated Nanoprobes for Optical/MRI/CT Triple-Modal in Vivo Tumor Imaging. Nanoscale 2017, 9, 4620–4628. [Google Scholar] [CrossRef]
- Pan, U.N.; Khandelia, R.; Sanpui, P.; Das, S.; Paul, A.; Chattopadhyay, A. Protein-Based Multifunctional Nanocarriers for Imaging, Photothermal Therapy, and Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 19495–19501. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Du, T.; ur Rehman, F.; Lai, L.; Liu, X.; Jiang, X.; Li, X.; Chen, Y.; Zhang, H.; Sun, Y.; et al. Biosynthesized Gold Nanoclusters and Iron Complexes as Scaffolds for Multimodal Cancer Bioimaging. Small 2016, 12, 6255–6265. [Google Scholar] [CrossRef]
- Pahari, S.K.; Olszakier, S.; Kahn, I.; Amirav, L. Magneto-Fluorescent Yolk–Shell Nanoparticles. Chem. Mater. 2018, 30, 775–780. [Google Scholar] [CrossRef]
- Su, X.; Xu, Y.; Che, Y.; Liao, X.; Jiang, Y. A Type of Novel Fluorescent Magnetic Carbon Quantum Dots for Cells Imaging and Detection. J. Biomed. Mater. Res. A 2015, 103, 3956–3964. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yao, Y.; Song, Q. Gold Nanoclusters Decorated with Magnetic Iron Oxide Nanoparticles for Potential Multimodal Optical/Magnetic Resonance Imaging. J. Mater. Chem. C Mater. 2015, 3, 5910–5917. [Google Scholar] [CrossRef]
- Huang, C.-L.; Hsieh, W.-J.; Lin, C.-W.; Yang, H.-W.; Wang, C.-K. Multifunctional Liposomal Drug Delivery with Dual Probes of Magnetic Resonance and Fluorescence Imaging. Ceram. Int. 2018, 44, 12442–12450. [Google Scholar] [CrossRef]
- Binaymotlagh, R.; Hajareh Haghighi, F.; Aboutalebi, F.; Mirahmadi-Zare, S.Z.; Hadadzadeh, H.; Nasr-Esfahani, M.-H. Selective Chemotherapy and Imaging of Colorectal and Breast Cancer Cells by a Modified MUC-1 Aptamer Conjugated to a Poly(Ethylene Glycol)-Dimethacrylate Coated Fe3O4–AuNCs Nanocomposite. New J. Chem. 2019, 43, 238–248. [Google Scholar] [CrossRef]
- Sheng, J.; Jiang, X.; Wang, L.; Yang, M.; Liu, Y.-N. Biomimetic Mineralization Guided One-Pot Preparation of Gold Clusters Anchored Two-Dimensional MnO2 Nanosheets for Fluorometric/Magnetic Bimodal Sensing. Anal. Chem. 2018, 90, 2926–2932. [Google Scholar] [CrossRef]
- Xu, Y.; Palchoudhury, S.; Qin, Y.; Macher, T.; Bao, Y. Make Conjugation Simple: A Facile Approach to Integrated Nanostructures. Langmuir 2012, 28, 8767–8772. [Google Scholar] [CrossRef]
- Meng, L.; Ma, X.; Jiang, S.; Ji, G.; Han, W.; Xu, B.; Tian, J.; Tian, W. High-Efficiency Fluorescent and Magnetic Multimodal Probe for Long-Term Monitoring and Deep Penetration Imaging of Tumors. J. Mater. Chem. B 2019, 7, 5345–5351. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-L.; Tan, J.-E.; Tian, Y.; Huang, S.; Sun, P.-H.; Wang, M.; Han, Y.-J.; Li, H.-S.; Wu, H.-B.; Zhang, X.-M.; et al. Multifunctional Superparamagnetic Nanoparticles Conjugated with Fluorescein-Labeled Designed Ankyrin Repeat Protein as an Efficient HER2-Targeted Probe in Breast Cancer. Biomaterials 2017, 147, 86–98. [Google Scholar] [CrossRef]
- Le, W.; Cui, S.; Chen, X.; Zhu, H.; Chen, B.; Cui, Z. Facile Synthesis of Gd-Functionalized Gold Nanoclusters as Potential MRI/CT Contrast Agents. Nanomaterials 2016, 6, 65. [Google Scholar] [CrossRef] [Green Version]
- Liang, G.; Xiao, L. Gd 3+-Functionalized Gold Nanoclusters for Fluorescence–Magnetic Resonance Bimodal Imaging. Biomater. Sci. 2017, 5, 2122–2130. [Google Scholar] [CrossRef]
- Dong, D.; Jing, X.; Zhang, X.; Hu, X.; Wu, Y.; Duan, C. Gadolinium(III)–Fluorescein Complex as a Dual Modal Probe for MRI and Fluorescence Zinc Sensing. Tetrahedron 2012, 68, 306–310. [Google Scholar] [CrossRef]
- Guan, S.; Liang, R.; Li, C.; Wei, M. A Supramolecular Material for Dual-Modal Imaging and Targeted Cancer Therapy. Talanta 2017, 165, 297–303. [Google Scholar] [CrossRef]
- Andrýsková, P.; Šišková, K.M.; Michetschlägerová, Š.; Jiráková, K.; Kubala, M.; Jirák, D. The Effect of Fatty Acids and BSA Purity on Synthesis and Properties of Fluorescent Gold Nanoclusters. Nanomaterials 2020, 10, 343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, N.-Y.; Lin, Y.-W. Microwave-Assisted Synthesis of Bovine Serum Albumin–Gold Nanoclusters and Their Fluorescence-Quenched Sensing of Hg2+ Ions. New J. Chem. 2016, 40, 1155–1161. [Google Scholar] [CrossRef]
- Yan, L.; Cai, Y.; Zheng, B.; Yuan, H.; Guo, Y.; Xiao, D.; Choi, M.M.F. Microwave-Assisted Synthesis of BSA-Stabilized and HSA-Protected Gold Nanoclusters with Red Emission. J. Mater. Chem. 2012, 22, 1000–1005. [Google Scholar] [CrossRef]
- Ostruszka, R.; Zoppellaro, G.; Tomanec, O.; Pinkas, D.; Filimonenko, V.; Šišková, K. Evidence of Au(II) and Au(0) States in Bovine Serum Albumin-Au Nanoclusters Revealed by CW-EPR/LEPR and Peculiarities in HR-TEM/STEM Imaging. Nanomaterials 2022, 12, 1425. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yang, L.; Gao, J.; Chen, X. Structure-Relaxivity Relationships of Magnetic Nanoparticles for Magnetic Resonance Imaging. Adv. Mater. 2019, 31, 1804567. [Google Scholar] [CrossRef] [PubMed]
- Babes, L.; Denizot, B.; Tanguy, G.; le Jeune, J.J.; Jallet, P. Synthesis of Iron Oxide Nanoparticles Used as MRI Contrast Agents: A Parametric Study. J. Colloid Interface Sci. 1999, 212, 474–482. [Google Scholar] [CrossRef]
- Bajaj, A.; Samanta, B.; Yan, H.; Jerry, D.J.; Rotello, V.M. Stability, Toxicity and Differential Cellular Uptake of Protein Passivated-Fe3O4 Nanoparticles. J. Mater. Chem. 2009, 19, 6328. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Hua, M.; Fang, K.; Liang, R. BSA Directed-Synthesis of Biocompatible Fe3O4 Nanoparticles for Dual-Modal T1 and T2 MR Imaging in Vivo. Anal. Methods 2017, 9, 3099–3104. [Google Scholar] [CrossRef]
- Nosrati, H.; Sefidi, N.; Sharafi, A.; Danafar, H.; Kheiri Manjili, H. Bovine Serum Albumin (BSA) Coated Iron Oxide Magnetic Nanoparticles as Biocompatible Carriers for Curcumin-Anticancer Drug. Bioorg. Chem. 2018, 76, 501–509. [Google Scholar] [CrossRef]
- Xu, S.; Wang, J.; Wei, Y.; Zhao, H.; Tao, T.; Wang, H.; Wang, Z.; Du, J.; Wang, H.; Qian, J.; et al. In Situ One-Pot Synthesis of Fe2O3@BSA Core-Shell Nanoparticles as Enhanced T1-Weighted Magnetic Resonance Imagine Contrast Agents. ACS Appl. Mater. Interfaces 2020, 12, 56701–56711. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Chang, Y.; Zhao, L.; Zhang, K.; Zhao, Y.; Gao, F.; Gao, X. Ultrasmall Superparamagnetic Iron Oxide Nanoparticle for T2-Weighted Magnetic Resonance Imaging. ACS Appl. Mater. Interfaces 2017, 9, 28959–28966. [Google Scholar] [CrossRef]
- Li, H.; Yan, K.; Shang, Y.; Shrestha, L.; Liao, R.; Liu, F.; Li, P.; Xu, H.; Xu, Z.; Chu, P.K. Folate-Bovine Serum Albumin Functionalized Polymeric Micelles Loaded with Superparamagnetic Iron Oxide Nanoparticles for Tumor Targeting and Magnetic Resonance Imaging. Acta Biomater. 2015, 15, 117–126. [Google Scholar] [CrossRef]
- Vismara, E.; Bongio, C.; Coletti, A.; Edelman, R.; Serafini, A.; Mauri, M.; Simonutti, R.; Bertini, S.; Urso, E.; Assaraf, Y.; et al. Albumin and Hyaluronic Acid-Coated Superparamagnetic Iron Oxide Nanoparticles Loaded with Paclitaxel for Biomedical Applications. Molecules 2017, 22, 1030. [Google Scholar] [CrossRef] [Green Version]
- An, L.; Yan, C.; Mu, X.; Tao, C.; Tian, Q.; Lin, J.; Yang, S. Paclitaxel-Induced Ultrasmall Gallic Acid-Fe@BSA Self-Assembly with Enhanced MRI Performance and Tumor Accumulation for Cancer Theranostics. ACS Appl. Mater. Interfaces 2018, 10, 28483–28493. [Google Scholar] [CrossRef]
- Tian, Q.; An, L.; Tian, Q.; Lin, J.; Yang, S. Ellagic Acid-Fe@BSA Nanoparticles for Endogenous H2S Accelerated Fe(III)/Fe(II) Conversion and Photothermal Synergistically Enhanced Chemodynamic Therapy. Theranostics 2020, 10, 4101–4115. [Google Scholar] [CrossRef]
- Harini, G.; Balasurya, S.; Khan, S.S. Recent Advances on Gadolinium-Based Nano-Photocatalysts for Environmental Remediation and Clean Energy Production: Properties, Fabrication, Defect Engineering and Toxicity. J. Clean Prod. 2022, 345, 131139. [Google Scholar] [CrossRef]
- Gao, F.; Qu, H.; Duan, Y.; Wang, J.; Song, X.; Ji, T.; Cao, L.; Nie, G.; Sun, S. Dopamine Coating as a General and Facile Route to Biofunctionalization of Superparamagnetic Fe3O4 Nanoparticles for Magnetic Separation of Proteins. RSC Adv. 2014, 4, 6657. [Google Scholar] [CrossRef]
- Nosrati, H.; Davaran, S.; Kheiri Manjili, H.; Rezaeejam, H.; Danafar, H. Bovine Serum Albumin Stabilized Iron Oxide and Gold Bimetallic Heterodimers: Synthesis, Characterization and Stereological Study. Appl. Organomet. Chem. 2019, 33, e5155. [Google Scholar] [CrossRef]
- Nosrati, H.; Baghdadchi, Y.; Abbasi, R.; Barsbay, M.; Ghaffarlou, M.; Abhari, F.; Mohammadi, A.; Kavetskyy, T.; Bochani, S.; Rezaeejam, H.; et al. Iron Oxide and Gold Bimetallic Radiosensitizers for Synchronous Tumor Chemoradiation Therapy in 4T1 Breast Cancer Murine Model. J. Mater. Chem. B 2021, 9, 4510–4522. [Google Scholar] [CrossRef] [PubMed]
- Rurack, K.; Spieles, M. Fluorescence Quantum Yields of a Series of Red and Near-Infrared Dyes Emitting at 600−1000 nm. Anal. Chem. 2011, 83, 1232–1242. [Google Scholar] [CrossRef]
- Procházka, V.; Novák, P.; Stejskal, A. Department of Experimental Physics. Mössbauer Spectrometers OLTWINS. Available online: http://oltwins.upol.cz/ (accessed on 6 February 2023).
- Klencsár, Z.; Kuzmann, E.; Vértes, A. User-Friendly Software for Mössbauer Spectrum Analysis. J. Radioanal. Nucl. Chem. Artic. 1996, 210, 105–118. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, C.; Razzaque, S.; Hussain, I.; Lu, Q.-W.; Tan, B. Synthesis of Water-Soluble and Highly Fluorescent Gold Nanoclusters for Fe3+ Sensing in Living Cells Using Fluorescence Imaging. J. Mater. Chem. B 2017, 5, 5608–5615. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Jiang, H.; Wang, X. Selective and Sensitive Detection of Fe3+ Ion in Drinking Water Using L-Glutathione Stabilized Red Fluorescent Gold Nanoclusters. J. Nanosci. Nanotechnol. 2016, 16, 12179–12186. [Google Scholar] [CrossRef]
- Ungor, D.; Csapó, E.; Kismárton, B.; Juhász, Á.; Dékány, I. Nucleotide-Directed Syntheses of Gold Nanohybrid Systems with Structure-Dependent Optical Features: Selective Fluorescence Sensing of Fe3+ Ions. Colloids Surf B Biointerfaces 2017, 155, 135–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šišková, K.; Machala, L.; Tuček, J.; Kašlík, J.; Mojzeš, P.; Zbořil, R. Mixtures of L-Amino Acids as Reaction Medium for Formation of Iron Nanoparticles: The Order of Addition into a Ferrous Salt Solution Matters. Int. J. Mol. Sci. 2013, 14, 19452–19473. [Google Scholar] [CrossRef] [Green Version]
- Rohrer, M.; Bauer, H.; Mintorovitch, J.; Requardt, M.; Weinmann, H.-J. Comparison of Magnetic Properties of MRI Contrast Media Solutions at Different Magnetic Field Strengths. Investig. Radiol. 2005, 40, 715–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanisz, G.J.; Henkelman, R.M. Gd-DTPA Relaxivity Depends on Macromolecular Content. Magn. Reson. Med. 2000, 44, 665–667. [Google Scholar] [CrossRef]
- Bjørnerud, A.; Johansson, L.O.; Briley-Saebø, K.; Ahlström, H.K. Assessment of T1 and T2* Effects in Vivo and Ex Vivo Using Iron Oxide Nanoparticles in Steady State-Dependence on Blood Volume and Water Exchange. Magn. Reson. Med. 2002, 47, 461–471. [Google Scholar] [CrossRef]
- Van Osch, M.J.P.; Vonken, E.P.A.; Viergever, M.A.; van der Grond, J.; Bakker, C.J.G. Measuring the Arterial Input Function with Gradient Echo Sequences. Magn. Reson. Med. 2003, 49, 1067–1076. [Google Scholar] [CrossRef]
- Zhao, J.M.; Clingman, C.S.; Närväinen, M.J.; Kauppinen, R.A.; van Zijl, P.C.M. Oxygenation and Hematocrit Dependence of Transverse Relaxation Rates of Blood at 3T. Magn. Reson. Med. 2007, 58, 592–597. [Google Scholar] [CrossRef]
- Calamante, F.; Connelly, A.; van Osch, M.J.P. Nonlinear ΔR2* Effects in Perfusion Quantification Using Bolus-Tracking MRI. Magn. Reson. Med. 2009, 61, 486–492. [Google Scholar] [CrossRef]
- Patil, V.; Jensen, J.H.; Johnson, G. Intravascular Contrast Agent T2* Relaxivity in Brain Tissue. NMR Biomed. 2013, 26, 392–399. [Google Scholar] [CrossRef] [Green Version]
- Ta, H.T.; Li, Z.; Wu, Y.; Cowin, G.; Zhang, S.; Yago, A.; Whittaker, A.K.; Xu, Z.P. Effects of Magnetic Field Strength and Particle Aggregation on Relaxivity of Ultra-Small Dual Contrast Iron Oxide Nanoparticles. Mater. Res. Express 2017, 4, 116105. [Google Scholar] [CrossRef]
- Weisskoff, R.; Zuo, C.S.; Boxerman, J.L.; Rosen, B.R. Microscopic Susceptibility Variation and Transverse Relaxation: Theory and Experiment. Magn. Reson. Med. 1994, 31, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Goegan, P.; Johnson, G.; Vincent, R. Effects of Serum Protein and Colloid on the AlamarBlue Assay in Cell Cultures. Toxicol. In Vitr. 1995, 9, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Funk, D.; Schrenk, H.-H.; Frei, E. Serum Albumin Leads to False-Positive Results in the XTT and the MTT Assay. Biotechniques 2007, 43, 178–186. [Google Scholar] [CrossRef]
- Neufeld, B.H.; Tapia, J.B.; Lutzke, A.; Reynolds, M.M. Small Molecule Interferences in Resazurin and MTT-Based Metabolic Assays in the Absence of Cells. Anal. Chem. 2018, 90, 6867–6876. [Google Scholar] [CrossRef] [PubMed]






| Sample | Z-Average [nm] | PDI |
|---|---|---|
| AuBSA | 23.9 ± 10.8 | 0.4 ± 0.1 |
| AuBSA-Fe | 71.2 ± 8.0 | 1.0 ± 0.0 |
| Fe Concentration [mM] | Relaxivity r1 [L·mmol−1·s−1] | Relaxivity r2 [L·mmol−1·s−1] |
|---|---|---|
| <0.52 | 0.41 ± 0.04 | 2.68 ± 0.11 |
| ≥0.52 | 0.39 ± 0.04 | 3.44 ± 0.36 |
| AuBSA-Fe | Average Viability [%] |
|---|---|
| Iron concentration < 0.52 mM | 78 ± 3 |
| Iron concentration ≥ 0.52 mM | 80 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ostruszka, R.; Půlpánová, D.; Pluháček, T.; Tomanec, O.; Novák, P.; Jirák, D.; Šišková, K. Facile One-Pot Green Synthesis of Magneto-Luminescent Bimetallic Nanocomposites with Potential as Dual Imaging Agent. Nanomaterials 2023, 13, 1027. https://doi.org/10.3390/nano13061027
Ostruszka R, Půlpánová D, Pluháček T, Tomanec O, Novák P, Jirák D, Šišková K. Facile One-Pot Green Synthesis of Magneto-Luminescent Bimetallic Nanocomposites with Potential as Dual Imaging Agent. Nanomaterials. 2023; 13(6):1027. https://doi.org/10.3390/nano13061027
Chicago/Turabian StyleOstruszka, Radek, Denisa Půlpánová, Tomáš Pluháček, Ondřej Tomanec, Petr Novák, Daniel Jirák, and Karolína Šišková. 2023. "Facile One-Pot Green Synthesis of Magneto-Luminescent Bimetallic Nanocomposites with Potential as Dual Imaging Agent" Nanomaterials 13, no. 6: 1027. https://doi.org/10.3390/nano13061027
APA StyleOstruszka, R., Půlpánová, D., Pluháček, T., Tomanec, O., Novák, P., Jirák, D., & Šišková, K. (2023). Facile One-Pot Green Synthesis of Magneto-Luminescent Bimetallic Nanocomposites with Potential as Dual Imaging Agent. Nanomaterials, 13(6), 1027. https://doi.org/10.3390/nano13061027