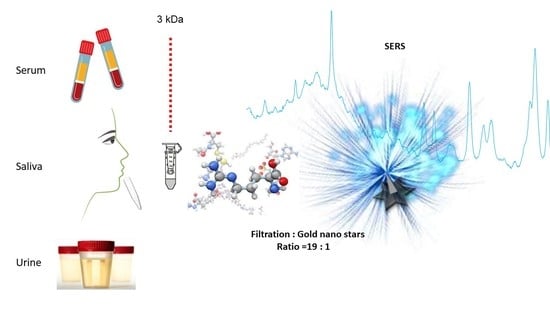
Surface-Enhanced Raman Analysis of Uric Acid and Hypoxanthine Analysis in Fractionated Bodily Fluids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gold Stars Synthesis and Characterisation
2.3. Fractionation of Biofluids by Centrifugation
2.4. HPLC
2.5. Sample Preparation for SERS Measurements
2.6. Spectroscopic Measurement
2.7. Data Analysis
3. Results
3.1. Characterisation of Gold Nanostars
3.2. HPLC and SERS of Diluted Series of Uric Acid and Hypoxanthine
3.3. SERS of 3 kDa Filtrate of Bodily Fluids
3.4. Overlapping Spectral Peaks of SERS of Uric Acid, Hypoxanthine and Body Fluids
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kanbay, M.; Solak, Y.; Dogan, E.; Lanaspa, M.A.; Covic, A. Uric Acid in Hypertension and Renal Disease: The Chicken or the Egg? Blood Purif. 2010, 30, 288–295. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Zeng, C. Update on the epidemiology, genetics, and therapeutic options of hyperuricemia. Am. J. Transl. Res. 2020, 12, 3167–3181. [Google Scholar] [PubMed]
- Vernerová, A.; Kujovská Krčmová, L.; Melichar, B.; Švec, F. Non-invasive determination of uric acid in human saliva in the diagnosis of serious disorders. Clin. Chem. Lab. Med. (CCLM) 2021, 59, 797–812. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Liu, J.; Zhang, H.; Jiang, M.; Jin, Y.; Zhang, M.; Hu, P. Improved ultra-high performance liquid chromatographic method for simultaneous determination of five gout-related metabolites in human serum. J. Sep. Sci. 2021, 44, 954–962. [Google Scholar] [CrossRef]
- Aliu, E.; Kanungo, S.; Arnold, G.L. Amino acid disorders. Ann. Transl. Med. 2018, 6, 471. [Google Scholar] [CrossRef]
- Bonnier, F.; Baker, M.J.; Byrne, H.J. Screening the Low Molecular Weight Fraction of Human Serum Using ATR-IR Spectroscopy Using ATR-IR Spectroscopy. J. Biophotonics 2016, 9, 1085–1097. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, M.S.; Leal, L.B.; Marcarini, W.D.; Pimentel, R.L.; Muller, M.; Vassallo, P.F.; Campos, L.C.G.; Dos Santos, L.; Luiz, W.B.; Mill, J.G. Rapid diagnosis of COVID-19 using FT-IR ATR spectroscopy and machine learning. Sci. Rep. 2021, 11, 15409. [Google Scholar] [CrossRef]
- e Silva, L.F.C.; Nogueira, M.S. New insights of Raman spectroscopy for oral clinical applications. Analyst 2018, 143, 6037–6048. [Google Scholar]
- Lu, Y.; Lin, L.; Ye, J. Human metabolite detection by surface-enhanced Raman spectroscopy. Mater. Today Bio 2022, 13, 100205. [Google Scholar] [CrossRef]
- Iancu, S.D.; Cozan, R.G.; Stefancu, A.; David, M.; Moisoiu, T.; Moroz-Dubenco, C.; Bajcsi, A.; Chira, C.; Andreica, A.; Leopold, L.F. SERS liquid biopsy in breast cancer. What can we learn from SERS on serum and urine? Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 273, 120992. [Google Scholar] [CrossRef] [PubMed]
- Bonnier, F.; Petitjean, F.; Baker, M.J.; Byrne, H.J. Improved protocols for vibrational spectroscopic analysis of body fluids. J. Biophotonics 2014, 7, 167–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.; Arellano, M.; Boontheung, P.; Wang, J.; Zhou, H.; Jiang, J.; Elashoff, D.; Wei, R.; Loo, J.A.; Wong, D.T. Salivary Proteomics for Oral Cancer Biomarker Discovery. Clin. Cancer Res. 2008, 14, 6246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calado, G.; Behl, I.; Daniel, A.; Byrne, H.J.; Lyng, F.M. Raman spectroscopic analysis of saliva for the diagnosis of oral cancer: A systematic review. Transl. Biophotonics 2019, 1, e201900001. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Yang, M.; Zhu, J.; Zhang, H.; Duan, Z.; Wang, S.; Liao, Z.; Liu, W. Developments in diagnostic applications of saliva in human organ diseases. Med. Nov. Technol. Devices 2022, 13, 100115. [Google Scholar] [CrossRef]
- Valpapuram, I.; Candeloro, P.; Coluccio, M.L.; Parrotta, E.I.; Giugni, A.; Das, G.; Cuda, G.; Di Fabrizio, E.; Perozziello, G. Waveguiding and SERS simplified Raman spectroscopy on biological samples. Biosensors 2019, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Kamińska, A.; Szymborski, T.; Jaroch, T.; Zmysłowski, A.; Szterk, A. Gold-capped silicon for ultrasensitive SERS-biosensing: Towards human biofluids analysis. Mater. Sci. Eng. C 2018, 84, 208–217. [Google Scholar] [CrossRef]
- Xiang, L.; Li, J.; Lin, J.; Lia, H. Determination of gouty arthritis’ biomarkers in human urine using reversed-phase high-performance liquid chromatography. J. Pharm. Anal. 2014, 4, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Westley, C.; Xu, Y.; Thilaganathan, B.; Carnell, A.J.; Turner, N.J.; Goodacre, R. Absolute Quantification of Uric Acid in Human Urine Using Surface Enhanced Raman Scattering with the Standard Addition Method. Anal. Chem. 2017, 89, 2472–2477. [Google Scholar] [CrossRef] [Green Version]
- Das, L.; Murthy, V.; Varma, A.K. Comprehensive Analysis of Low Molecular Weight Serum Proteome Enrichment for Mass Spectrometric Studies. ACS Omega 2020, 44, 28877–28888. [Google Scholar] [CrossRef]
- Bonifacio, A.; Marta, A.D.; Spizzo, R.; Cervo, S.; Steffan, A.; Colombatti, A.; Sergo, A. Surface-enhanced Raman spectroscopy of blood plasma and serum using Ag and Au nanoparticles: A systematic study. Anal. Bioanal. Chem. 2014, 406, 2355–2365. [Google Scholar] [CrossRef]
- Bonifacio, A.; Cervo, S.; Sergo, V. Label-free surface-enhanced Raman spectroscopy of biofluids: Fundamental aspects and diagnostic applications. Anal. Bioanal. Chem. 2015, 407, 8265–8277. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Bonnier, F.; Casey, A.; Shanahan, A.E.; Byrne, H.J. Surface enhanced Raman scattering with gold nanoparticles: Effect of particle shape. Anal. Methods 2014, 6, 9116–9123. [Google Scholar] [CrossRef] [Green Version]
- Bao, C.; Beziere, N.; del Pino, P.; Pelaz, B.; Estrada, G.; Tian, F.; Ntziachristos, V.; de la Fuente, J.M.; Cui, D. Gold nanoprisms as optoacoustic signal nanoamplifiers for in vivo bioimaging of gastrointestinal cancers. Small 2013, 9, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Chu, P.Y. Current and developing liquid biopsy techniques for breast cancer. Cancers 2022, 14, 2052. [Google Scholar] [CrossRef]
- Burt, J.L.; Gutiérrez-Wing, C.; Miki-Yoshida, M.; José-Yacamán, M. Noble-metal nanoparticles directly conjugated to globular proteins. Langmuir 2004, 20, 11778–11783. [Google Scholar] [CrossRef]
- Sen, T.; Haldar, K.K.; Patra, A. Au nanoparticle-based surface energy transfer probe for conformational changes of BSA protein. J. Phys. Chem. C 2008, 112, 17945–17951. [Google Scholar] [CrossRef]
- Shang, L.; Wang, Y.; Jiang, J.; Dong, S. pH-dependent protein conformational changes in albumin: Gold nanoparticle bioconjugates: A spectroscopic study. Langmuir 2007, 23, 2714–2721. [Google Scholar] [CrossRef]
- Treuel, L.; Malissek, M.; Grass, S.; Diendorf, J.; Mahl, D.; Meyer-Zaika, W.; Epple, M. Quantifying the influence of polymer coatings on the serum albumin corona formation around silver and gold nanoparticles. J. Nanopart. Res. 2012, 14, 1102. [Google Scholar] [CrossRef]
- Gebauer, J.S.; Malissek, M.; Simon, S.; Knauer, S.K.; Maskos, M.; Stauber, R.H.; Peukert, W.; Treuel, L. Impact of the nanoparticle--protein corona on colloidal stability and protein structure. Langmuir 2012, 28, 9673–9679. [Google Scholar] [CrossRef]
- Di Nardo, F.; Alladio, E.; Baggiani, C.; Cavalera, S.; Giovannoli, C.; Spano, G.; Anfossi, L. Colour-encoded lateral flow immunoassay for the simultaneous detection of aflatoxin B1 and type-B fumonisins in a single Test line. Talanta 2018, 192, 288–294. [Google Scholar] [CrossRef]
- Kaya, M.; Moriwaki, Y.; Ka, T.; Inokuchi, T.; Yamamoto, A.; Takahashi, S.; Tsutsumi, Z.; Tsuzita, J.; Oku, Y.; Yamamoto, T. Plasma concentrations and urinary excretion of purine bases (uric acid, hypoxanthine, and xanthine) and oxypurinol after rigorous exercise. Metabolism 2006, 55, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Casali, E.; Berni, P.; Spisni, A.; Baricchi, R.; Pertinhez, T.A. Hypoxanthine: A new paradigm to interpret the origin of transfusion toxicity. Blood Transfus. 2016, 14, 555–556. [Google Scholar] [PubMed]
- Ali, S.M.; Bonnier, F.; Tfayli, A.; Lambkin, H.; Flynn, K.; McDonagh, V.; Healy, C.; Clive Lee, T.; Lyng, F.M.; Byrne, H.J. Raman spectroscopic analysis of human skin tissue sections ex-vivo: Evaluation of the effects of tissue processing and dewaxing. J. Biomed. Opt. 2012, 18, 061202. [Google Scholar] [CrossRef] [Green Version]
- Udensi, J.; Loskutova, E.; Loughman, J.; Byrne, H.J. Quantitative Raman Analysis of Carotenoid Protein Complexes in Aqueous Solution. Molecules 2022, 27, 4724. [Google Scholar] [CrossRef]
- Turgan, N.; Boydak, B.; Habif, S.; Gülter, C.; Senol, B.; Mutaf, I.; Ozmen, D.; Bayindir, O. Urinary hypoxanthine and xanthine levels in acute coronary syndromes. Int. J. Clin. Lab. Res. 1999, 29, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Maciejczy, K.M.; Nesterowicz, M.; Zalewska, A.; Biedrzycki, G.; Gerreth, P.; Hojan, K.; Gerreth, K. Salivary Xanthine Oxidase as a Potential Biomarker in Stroke Diagnostics. Front. Immunol. 2022, 13, 897413. [Google Scholar] [CrossRef] [PubMed]
- Podstawka, E.; Ozaki, Y.; Proniewicz, L.M. Part III: Surface-enhanced Raman scattering of amino acids and their homodipeptide monolayers deposited onto colloidal gold surface. Appl. Spectrosc. 2005, 59, 1516–1526. [Google Scholar] [CrossRef]
- Premasiri, W.R.; Lee, J.C.; Ziegler, L.D. Surface-Enhanced Raman Scattering of Whole Human Blood, Blood Plasma, and Red Blood Cells: Cellular Processes and Bioanalytical Sensing. J. Phys. Chem. B 2012, 116, 9376–9386. [Google Scholar] [CrossRef] [Green Version]
- Moisoiu, V.; Stefania, D.; Iancu, S.D.; Stefancu, A.; Moisoiu, T.; Pardini, B.; Dragomir, M.P.; Crisan, N.; Avram, L.; Crisan, D.; et al. SERS liquid biopsy: An emerging tool for medical diagnosis. Colloids Surf. B 2021, 208, 112064. [Google Scholar] [CrossRef]
- Parachalil, D.R.; McIntyre, J.; Byrne, H.J. Potential of Raman spectroscopy for the analysis of plasma/serum in the liquid state: Recent advances. Anal. Bioanal. Chem. 2020, 412, 1993–2007. [Google Scholar] [CrossRef] [Green Version]
- Esposito, A.; Bonifacio, A.; Sergo, V.; Fornasaro, S. Label-free Surface Enhanced Raman Scattering (SERS) on Centrifugal Silver Plasmonic Paper (CSPP): A Novel Methodology for Unprocessed Biofluids Sampling and Analysis. Biosensors 2021, 11, 467. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.A.; Dina, N.E.; Cheng, H.; Valev, V.K.; Zhang, L. Surface-enhanced Raman spectroscopy for bioanalysis and diagnosis. Nanoscale 2021, 13, 11593–11634. [Google Scholar] [CrossRef]
- Keuleers, R.; Desseyn, H.O.; Rousseau, B.; Van Alsenoy, C. Vibrational Analysis of Urea. J. Phys. Chem. A 1999, 103, 462. [Google Scholar] [CrossRef]
- Kao, Y.C.; Han, X.; Lee, Y.H.; Lee, H.K.; Phan-Quang, G.C.; Lay, C.L.; Sim, H.Y.F.; Phua, V.J.X.; Ng, L.S.; Ku, C.W. Others 2020 Multiplex surface-enhanced Raman scattering identification and quantification of urine metabolites in patient samples within 30 min. ACS Nano 2020, 14, 2542–2552. [Google Scholar] [CrossRef] [PubMed]
- Daudon, M.; Protat, M.F.; Reveillaud, R.J.; Jaeschke-Boyer, H. Infrared spectrometry and Raman microprobe in the analysis of urinary calculi. Kidney Int. 1983, 23, 842–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarigul, N.; Korkmaz, F.; Kurultak, İ.A. New Artificial Urine Protocol to Better Imitate Human Urine. Sci Rep. 2019, 9, 20159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menon, P.S.; Said, F.A.; Mei, G.S.; Berhanuddin, D.D.; Umar, A.A.; Shaari, S.; Majlis, B.Y. Urea and creatinine detection on nano-laminated gold thin film using Kretschmann-based surface plasmon resonance biosensor. PLoS ONE 2018, 13, e0201228. [Google Scholar] [CrossRef] [Green Version]
- Bakar, N.A.; Salleh, M.M.; Umar, A.A. Detection of creatinine on triangular silver nanoplates surface using surface-enhance Raman scattering sensor. Int. J. Biomed. Nanosci. Nanotechnol. 2017, 3, 335–342. [Google Scholar] [CrossRef]
- Bhatia, P.; Gupta, B.D. Fabrication and characterization of a surface plasmon resonance based fiber optic urea sensor for biomedical applications. Sens. Actuators B Chem. 2012, 161, 434–438. [Google Scholar] [CrossRef]
- Raveendran, J.; Resmi, P.E.; Ramachandran, T.; Nair, B.G.; Satheesh Babu, T.G. Fabrication of a disposable non-enzymatic electrochemical creatinine sensor. Sens. Actuators B Chem. 2017, 243, 589–59547. [Google Scholar] [CrossRef]
- Byrne, H.; Bonnier, F.; McIntyre, J.; Parachalil, D.R. Quantitative analysis of human blood serum using vibrational spectroscopy. Clin. Spectrosc. 2020, 2, 100004. [Google Scholar] [CrossRef]
- Elumalai, B.; Rajasekaran, R.; Aruna, P.; Koteeswaran, D.; Ganesan, S. Discrimination of premalignant conditions of oral cancer using Raman spectroscopy of urinary metabolites. In Proceedings of the SPIE, Optical Biopsy XIII: Toward Real-Time Spectroscopic Imaging and Diagnosis, San Francisco, CA, USA, 7–12 February 2015; Alfano, R.R., Demos, S.G., Eds.; Volume 9318, pp. 74–80. [Google Scholar]
- Derruau, S.; Robinet, J.; Untereiner, V.; Piot, O.; Sockalingum, G.D.; Lorimier, S. Vibrational Spectroscopy Saliva Profiling as Biometric Tool for Disease Diagnostics: A Systematic Literature Review. Molecules 2020, 25, 4142. [Google Scholar] [CrossRef] [PubMed]
- Bandhakavi, S.; Stone, M.D.; Onsongo, G.; Van Riper, S.K.; Griffin, T.J. A dynamic range compression and three-dimensional peptide fractionation analysis platform expands proteome coverage and the diagnostic potential of whole saliva. J. Proteome Res. 2009, 8, 5590–5600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiappin, S.; Antonelli, G.; Gatti, R.; Elio, F. Saliva specimen: A new laboratory tool for diagnostic and basic investigation. Clin. Chim. Acta 2007, 383, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Aitekenov, S.; Sultangaziyev, A.; Abdirova, P.; Yussupova, L.; Gaipov, A.; Utegulov, Z.; Bukasov, R. Raman, Infrared and Brillouin spectroscopies of biofluids for medical diagnostics and for detection of biomarkers. Crit. Rev. Anal. Chem. 2022, 2022, 1–30. [Google Scholar]
- Haroon, M.; Tahir, M.; Nawaz, H.; Majeed, M.I.; Al-Saadi, A.A. Surface-Enhanced Raman Scattering (SERS) Spectroscopy for Prostate Cancer Diagnosis. A Review. Photodiagn. Photodyn. Ther. 2021, 37, 102690. [Google Scholar] [CrossRef]
- Stefancu, A.; Badarinza, M.; Moisoiu, V.; Iancu, S.D.; Serban, O.; Leopold, N.; Fodor, D. SERS-based liquid biopsy of saliva and serum from patients with Sjögren’s syndrome. Anal. Bioanal. Chem. 2019, 411, 5877–5883. [Google Scholar] [CrossRef]
- Gurian, E.; Giraudi, P.; Rosso, N.; Tiribelli, C.; Bonazza, D.; Zanconati, F.; Giuricin, M.; Palmisano, S.; de Manzini, N.; Sergo, V.; et al. Differentiation between stages of non-alcoholic fatty liver diseases using surface-enhanced Raman spectroscopy. Anal. Chim. Acta 2020, 1110, 190–198. [Google Scholar] [CrossRef]
- Rickard, J.J.S.; Di-Pietro, V.; Smith, D.J.; Davies, D.J.; Belli, A.; Oppenheimer, P.G. Rapid optofluidic detection of biomarkers for traumatic brain injury via surface-enhanced Raman spectroscopy. Nat. Biomed. Eng. 2020, 4, 610–623. [Google Scholar] [CrossRef]
- Panikar, S.S.; Cialla-May, D.; De la Rosa, E.; Salasl, P.; Popp, J. Towards translation of surface-enhanced Raman spectroscopy (SERS) to clinical practice: Progress and trends. Trends Analyt. Chem. 2021, 134, 116122. [Google Scholar] [CrossRef]
- Dina, N.E.; Tahir, M.A.; Bajwa, S.Z.; Amin, I.; Valev, V.K.; Zhang, L. SERS-based antibiotic susceptibility testing: Towards point-of-care clinical diagnosis. Biosens. Bioelectron. 2023, 219, 114843. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, C.A.; Lewis, P.D.; Dunstan, P.R.; Harris, D.A. Role of Raman spectroscopy and surface enhanced Raman spectroscopy in colorectal cancer. World J. Gastrointest. Oncol. 2016, 8, 427. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Pijuan-Galito, S.; Rho, H.S.; Vasilevich, A.S.; Eren, A.D.; Ge, L.; Habibović, P.; Alexander, M.R.; de Boer, J.; Carlier, A.; et al. High-Throughput Methods in the Discovery and Study of Biomaterials and Materiobiology. Chem. Rev. 2021, 121, 4561–4677. [Google Scholar] [CrossRef] [PubMed]
- Raynor, N.J.; Hallam, G.; Hynes, N.K.; Molloy, B.T. Blind to the risk: An analysis into the guidance offered to doctors and medical students with colour vision deficiency. Eye 2019, 33, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, L. Challenges for clinical implementation of Raman and FT-IR spectroscopy as a diagnostic tool. Photodiagn. Photodyn. Ther. 2020, 32, 101977. [Google Scholar] [CrossRef]








| SERS cm−1 | Assigment of Spectral Features | ||||
|---|---|---|---|---|---|
| Serum | Saliva | Urine | Uric Acid | Hypoxanthine | |
| 1620 | 1620 | 1620 | ν ring vibration [35] | − | + |
| 1560 | 1560 | 1560 | vibration C−N [10] | + | − |
| 1436 | 1436 | 1436 | (C2 H2) [10] | + | − |
| 1343 | 1343 | 1343 | ν ring vibration [3,36] | + | − |
| 1290 | 1290 | 1290 | (C2 H2) [36] | − | + |
| 1206 | 1206 | 1206 | C = O vibration [37] | + | + |
| 1086 | 1086 | 1086 | (NH3), (CH2) [10] | + | − |
| 1000 | 1000 | 1000 | symmetric C-N stretching [10] | + | − |
| 860 | 860 | 860 | side chain vibrations [37] | − | + |
| 802 | 802 | 802 | (CH2) [38] | + | + |
| 712 | 712 | 712 | N−H bending [36] | + | − |
| 640 | 640 | 640 | skeletal ring deformation [35] | + | − |
| 560 | 560 | 560 | ring vibration [37] | − | + |
| 442 | 442 | 442 | (NH2) [10] | + | − |
| SERS | HPLC | ||||
|---|---|---|---|---|---|
| Serum | Saliva | Urine | Serum | Saliva | Urine |
| 8 | 23 | 18 | 7 | 21 | 17 |
| 10 | 21 | 10 | 11 | 20 | 12 |
| 13 | 15 | 20 | 11 | 17 | 23 |
| 9 | 12 | 11 | 8 | 13 | 10 |
| 17 | 22 | 22 | 19 | 23 | 20 |
| SERS | HPLC | ||||
|---|---|---|---|---|---|
| Serum | Saliva | Urine | Serum | Saliva | Urine |
| 43 | 53 | 67 | 47 | 51 | 61 |
| 37 | 57 | 78 | 41 | 50 | 72 |
| 33 | 47 | 170 | 31 | 47 | 153 |
| 55 | 47 | 70 | 58 | 43 | 70 |
| 53 | 52 | 87 | 59 | 53 | 85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, F.; Carvalho, L.F.d.C.e.S.d.; Casey, A.; Nogueira, M.S.; Byrne, H.J. Surface-Enhanced Raman Analysis of Uric Acid and Hypoxanthine Analysis in Fractionated Bodily Fluids. Nanomaterials 2023, 13, 1216. https://doi.org/10.3390/nano13071216
Tian F, Carvalho LFdCeSd, Casey A, Nogueira MS, Byrne HJ. Surface-Enhanced Raman Analysis of Uric Acid and Hypoxanthine Analysis in Fractionated Bodily Fluids. Nanomaterials. 2023; 13(7):1216. https://doi.org/10.3390/nano13071216
Chicago/Turabian StyleTian, Furong, Luis Felipe das Chagas e Silva de Carvalho, Alan Casey, Marcelo Saito Nogueira, and Hugh J. Byrne. 2023. "Surface-Enhanced Raman Analysis of Uric Acid and Hypoxanthine Analysis in Fractionated Bodily Fluids" Nanomaterials 13, no. 7: 1216. https://doi.org/10.3390/nano13071216
APA StyleTian, F., Carvalho, L. F. d. C. e. S. d., Casey, A., Nogueira, M. S., & Byrne, H. J. (2023). Surface-Enhanced Raman Analysis of Uric Acid and Hypoxanthine Analysis in Fractionated Bodily Fluids. Nanomaterials, 13(7), 1216. https://doi.org/10.3390/nano13071216