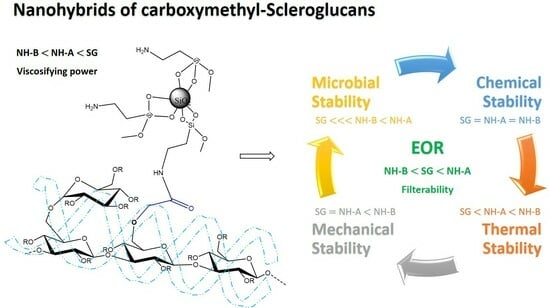
Evaluation of the Thermal, Chemical, Mechanical, and Microbial Stability of New Nanohybrids Based on Carboxymethyl-Scleroglucan and Silica Nanoparticles for EOR Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Biopolymer Solutions Preparation
2.2.2. Viscosity Measurements
2.2.3. Rheological Behavior
2.2.4. Filter Ratio (FR)
2.2.5. Thermal Stability
2.2.6. Mechanical Stability
2.2.7. Chemical Stability: Effect of pH and Electrolytes
2.2.8. Microbial Stability: Anaerobic Biodegradation Tests
3. Results
3.1. Rheological Behavior
3.2. Filter Ratio (FR)
3.3. Mechanical Stability
3.4. Chemical Stability: Effect of pH and Electrolytes
3.5. Microbial Stability: Anaerobic Biodegradation Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liang, K.; Han, P.; Chen, Q.; Su, X.; Feng, Y. Comparative study on enhancing oil recovery under high temperature and high salinity: Polysaccharides versus synthetic polymer. ACS Omega 2019, 4, 10620–10628. [Google Scholar] [CrossRef] [PubMed]
- Kozlowicz, B.; Muhammed, F.; Kadhum, M.; Khambete, M.; Jensen, T.; Sumner, E.; Ravikiran, R.; Ray, C. Qualification and field injection of scleroglucan. In Proceedings of the IOR 2019–20th European Symposium on Improved Oil Recovery, Pau, France, 8–10 April 2019; SPE; European Association of Geoscientists & Engineers: Utrecht, The Netherlands, 2019. [Google Scholar]
- Jensen, T.; Kadhum, M.; Kozlowicz, B.; Sumner, E.S.; Malsam, J.; Muhammed, F.; Ravikiran, R. Chemical EOR under harsh conditions: Scleroglucan as a viable commercial solution. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 14–18 April 2018; SPE; OnePetro: Richardson, TX, USA, 2018. [Google Scholar]
- Castro-García, R.-H.; Gallo, S.L.; Ardila, J.L.R.; Pérez, H.I.Q.; Ventura, E.J.M.; Arango, J.F.Z. Heavy oil and high-temperature polymer EOR applications. CTF-Cienc. Tecnol. Y Futuro 2020, 10, 73–83. [Google Scholar] [CrossRef]
- Rivenq, R.C.; Donche, A.; Nolk, C. Nolk, Improved scleroglucan for polymer flooding under harsh reservoir conditions. SPE Reserv. Eng. 1992, 7, 15–20. [Google Scholar] [CrossRef]
- Davison, P.; Mentzer, E. Polymer flooding in North Sea reservoirs. Soc. Pet. Eng. J. 1982, 22, 353–362. [Google Scholar] [CrossRef]
- Kozlowicz, B.; Jensen, T.; Khambete, M.; Kadhum, M.J.; Garshol, F.K.; Vik, E.A.; Tomczak-Wandzel, R.; Wever, D.A.; Lomans, B.P.; Ray, C. Scleroglucan Polymer Stability: Thermal, Chemical, and Microbial. In Proceedings of the SPE Improved Oil Recovery Conference, Virtual, 31 August–4 September 2020; SPE; Society of Petroleum Engineers: Richardson, TX, USA, 2020. [Google Scholar]
- Corredor, L.M.; Aliabadian, E.; Husein, M.; Chen, Z.; Maini, B.; Sundararaj, U. Heavy oil recovery by surface modified silica nanoparticle/HPAM nanofluids. Fuel 2019, 252, 622–634. [Google Scholar] [CrossRef]
- Franco, C.A.; Franco, C.A.; Zabala, R.D.; Bahamón, I.; Forero, A.; Cortés, F.B. Field Applications of Nanotechnology in the Oil and Gas Industry: Recent Advances and Perspectives. Energy Fuels 2021, 35, 19266–19287. [Google Scholar] [CrossRef]
- Castro, R.H.; Corredor, L.M.; Burgos, I.; Llanos, S.; Franco, C.A.; Cortés, F.B.; Idrobo, E.A.; Romero Bohórquez, A.R. Synthesis and Characterization of New Nanohybrids based on Carboxymethyl-Scleroglucan and Silica Nanoparticles for EOR processes. Nanomaterials 2024, 22, 13. [Google Scholar]
- Giraldo, L.J.; Franco, C.A.; Llanos, S.; Cortés, F.B. Synergy of SiO2 nanoparticle-polymer in enhanced oil recovery process to avoid formation damage caused by retention in porous media and improve resistance to degradative effects. In Formation Damage in Oil and Gas Reservoirs: Nanotechnology Applications for Its Inhibition/Remediation; Nova Science Publishers: Hapog, NY, USA, 2018; Volume 46. [Google Scholar]
- Llanos, S.; Giraldo, L.J.; Santamaria, O.; Franco, C.A.; Cortés, F.B. Effect of sodium oleate surfactant concentration grafted onto SiO2 nanoparticles in polymer flooding processes. ACS Omega 2018, 3, 18673–18684. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, L.J.; Giraldo, M.A.; Llanos, S.; Maya, G.; Zabala, R.D.; Nassar, N.N.; Franco, C.A.; Alvarado, V.; Cortés, F.B. The effects of SiO2 nanoparticles on the thermal stability and rheological behavior of hydrolyzed polyacrylamide based polymeric solutions. J. Pet. Sci. Eng. 2017, 159, 841–852. [Google Scholar] [CrossRef]
- Ruiz-Cañas, M.C.; Manrique, E.; Romero, A.; Quintero, H. Hybrid Nanomaterials for Enhanced Oil Recovery: New Alternatives for Polymer Thermal Degradation. In Proceedings of the SPE Latin American and Caribbean Petroleum Engineering Conference, Rio de Janeiro, Brazil, 18–19 May 2020; SPE; Society of Petroleum Engineers: Richardson, TX, USA, 2020. [Google Scholar]
- Ruiz-Cañas, M.C.; Quintero, H.I.; Corredor, L.M.; Manrique, E.; Bohórquez, A.R.R. New Nanohybrid Based on Hydrolyzed Polyacrylamide and Silica Nanoparticles: Morphological, Structural and Thermal Properties. Polymers 2020, 12, 1152. [Google Scholar] [CrossRef]
- Corredor, L.M.; Espinosa, C.; Delgadillo, C.L.; Llanos, S.; Castro, R.H.; Quintero, H.I.; Cañas, M.C.R.; Bohorquez, A.R.R.; Manrique, E. Flow Behavior through Porous Media and Displacement Performance of a SILICA/PAM Nanohybrid: Experimental and Numerical Simulation Study. ACS Omega 2024, 9, 7923–7936. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.H.; Burgos, I.; Corredor, L.M.; Llanos, S.; Franco, C.A.; Cortés, F.B.; Bohórquez, A.R.R. Carboxymethyl Scleroglucan Synthesized via O-Alkylation Reaction with Different Degrees of Substitution: Rheology and Thermal Stability. Polymers 2024, 16, 207. [Google Scholar] [CrossRef] [PubMed]
- Castillo, N.A.; Valdez, A.L.; Fariña, J.I. Microbial production of scleroglucan and downstream processing. Front. Microbiol. 2015, 6, 145963. [Google Scholar] [CrossRef] [PubMed]
- Abraham, T.W.; Sumner, E.S. Method for Solubilizing Biopolymer Solids for Enhanced oil Recovery Applications. Google Patents WO2017172707A1, 28 March 2019. [Google Scholar]
- Yasuda, K. Investigation of the Analogies between Viscometric and Linear Viscoelastic Properties of Polystyrene Fluids. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1979. [Google Scholar]
- Osswald, T.; Rudolph, N. Polymer Rheology; Carl Hanser: München, Germany, 2015. [Google Scholar]
- Carreau, P.J. Rheological equations from molecular network theories. Trans. Soc. Rheol. 1972, 16, 99–127. [Google Scholar] [CrossRef]
- American Petroleum Institute. Production Department. Recommended Practices for Evaluation of Polymers Used in Enhanced Oil Recovery Operations; American Petroleum Institute: Washington, DC, USA, 1990. [Google Scholar]
- Rodríguez-Mateus, Z.-P.; Angarita, R.C.; Niño-Gómez, J.A.; Corredor, L.M.; Llanos Gallo, S.; Quintero, H.; Castro-García, R.H. Biodegradation and toxicity of scleroglucan for enhanced oil recovery. CTF-Cienc. Tecnol. Y Futuro 2022, 12, 5–12. [Google Scholar] [CrossRef]
- TM0194-2004; Standard Test Method–Field Monitoring of Bacterial Growth in Oil and Gas Systems. National Association of Corrosion Engineers International: Houston, TX, USA, 2004.
- Jouenne, S. Polymer flooding in high temperature, high salinity conditions: Selection of polymer type and polymer chemistry, thermal stability. J. Pet. Sci. Eng. 2020, 195, 107545. [Google Scholar] [CrossRef]
- Castro, R.H.; Corredor, L.M.; Llanos, S.; Causil, M.A.; Arias, A.; Pérez, E.; Quintero, H.I.; Bohórquez, A.R.R.; Franco, C.A.; Cortés, F.B. Experimental Investigation of the Viscosity and Stability of Scleroglucan-Based Nanofluids for Enhanced Oil Recovery. Nanomaterials 2024, 14, 156. [Google Scholar] [CrossRef]
- Viñarta, S.C.; Delgado, O.D.; Figueroa, L.I.; Fariña, J.I. Effects of thermal, alkaline and ultrasonic treatments on scleroglucan stability and flow behavior. Carbohydr. Polym. 2013, 94, 496–504. [Google Scholar] [CrossRef]
- Salinas, E.R.; Bozich, J.S.; Kolbenschlag, S.; Kary-Heinrich, M.; Hopp, P.W.; Lukas, R.; Zok, S.; Hidding, B. Aquatic testing guidelines insufficiently control the influence of dilution water TOC and hardness on cationic polymer toxicity–a proposal to improve standardized test procedures. Chemosphere 2020, 259, 127473. [Google Scholar] [CrossRef]
- Survase, S.A.; Saudagar, P.S.; Bajaj, I.B.; Singhal, R.S. Scleroglucan: Fermentative production, downstream processing and applications. Food Technol. Biotechnol. 2007, 45, 107–118. [Google Scholar]
- Liu, Z.; Zhang, K.; Cheng, X. Cheng, Rheology of bacterial suspensions under confinement. Rheol. Acta 2019, 58, 439–451. [Google Scholar] [CrossRef]
- Hatwalne, Y.; Ramaswamy, S.; Rao, M.; Simha, R.A. Rheology of active-particle suspensions. Phys. Rev. Lett. 2004, 92, 118101. [Google Scholar] [CrossRef] [PubMed]
- Chui, J.Y.; Douarche, C.; Auradou, H.; Juanes, R. Rheology of bacterial superfluids in viscous environments. Soft Matter 2021, 17, 7004–7013. [Google Scholar] [CrossRef]
- Wilson, W.W.; Wade, M.M.; Holman, S.C.; Champlin, F.R. Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J. Microbiol. Methods 2001, 43, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, S.; Ronco, A.M.; Gotteland, R.M. ß-glucanos: ¿qué tipos existen y cuáles son sus beneficios en la salud? Rev. Chil. De Nutr. 2014, 41, 439–446. [Google Scholar] [CrossRef]
- Gutiérrez-Rojas, I.; Moreno-Sarmiento, N.; Montoya, D. Mecanismos y regulación de la hidrólisis enzimática de celulosa en hongos filamentosos: Casos clásicos y nuevos modelos. Rev. Iberoam. Micol. 2015, 32, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Elías, E.J.; McKinney, J.D. Carbon metabolism of intracellular bacteria. Cell. Microbiol. 2006, 8, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Mamonova, I.; Babushkina, I.V.; Norkin, I.A.; Gladkova, E.V.; Matasov, M.D.; Puchin’yan, D.M. Biological activity of metal nanoparticles and their oxides and their effect on bacterial cells. Nanotechnologies Russ. 2015, 10, 128–134. [Google Scholar] [CrossRef]
- Khalandi, B.; Asadi, N.; Milani, M.; Davaran, S.; Abadi, A.J.N.; Abasi, E.; Akbarzadeh, A. A review on potential role of silver nanoparticles and possible mechanisms of their actions on bacteria. Drug Res. 2017, 11, 70–76. [Google Scholar] [CrossRef]
- Selvarajan, V.; Obuobi, S.; Ee, P.L.R. Silica nanoparticles—A versatile tool for the treatment of bacterial infections. Front. Chem. 2020, 8, 602. [Google Scholar] [CrossRef]
- Pathak, V.M. Review on the current status of polymer degradation: A microbial approach. Bioresour. Bioprocess. 2017, 4, 1–31. [Google Scholar] [CrossRef]
- Arora, A.; Mishra, A. Antibacterial polymers–A mini review. Mater. Today Proc. 2018, 5, 17156–17161. [Google Scholar] [CrossRef]









| Sample | Abbreviation | Description |
|---|---|---|
| Sterile injection water with biopolymer | RSW/bio | Abiotic control in sterile injection water |
| Synthetic brine with biopolymer | SB/bio | Abiotic system in synthetic brine |
| Injection of water with biopolymer | RW/bio | Biotic control in injection water without biocide |
| Injection of water with biopolymer plus biocide | RW/bio/B | Biotic system in injection water with biocide |
| Injection water | RW | Biotic control without biopolymer |
| Parameter | SG | NH-A | NH-B |
|---|---|---|---|
| 0.4360 | 0.4801 | 0.4854 | |
| 22.9530 | 26.2618 | 37.7623 | |
| 0.0948 | 0.1619 | 0.2421 | |
| RMSE (%) | 2.66 | 3.59 | 4.58 |
| Sample | Abbreviation | Viscosity Change at 4/5 Weeks (%) |
|---|---|---|
| Sterile injection water with biopolymers (abiotic system) | RSW/SG | −4 |
| RSW/NH-A | +4 | |
| RSW/NH-B | +1 | |
| Synthetic brine with biopolymers (abiotic system) | SB/SG | −6 |
| SB/NH-A | +3 | |
| SB/NH-B | +1 | |
| Injection of water with biopolymers (biotic control) | RW/SG | −92 |
| RW/NH-A | +9 | |
| RW/NH-B | +9 | |
| Injection of water with biopolymers plus biocide (biotic system) | RW/SG/B | −32 |
| RW/NH-A/B | +28 | |
| RW/NH-B/B | +9 |
| Sample Type | Abbreviation | Total Growth of the Anaerobic Bacteria (Bacteria/mL) | ||
|---|---|---|---|---|
| 0 Weeks | 2–3 Weeks | 4–5 Weeks | ||
| Sterile injection water with biopolymer (abiotic control) | RSW/SG | <1.0 × 101 | <1.0 × 101 | <1.0 × 101 |
| RSW/NH-A | <1.0 × 101 | <1.0 × 101 | <1.0 × 101 | |
| RSW/NH-B | <1.0 × 101 | <1.0 × 101 | <1.0 × 101 | |
| Synthetic brine with biopolymer (abiotic system) | SB/SG | <1.0 × 101 | <1.0 × 101 | <1.0 × 101 |
| SB/NH-A | <1.0 × 101 | <1.0 × 101 | <1.0 × 101 | |
| SB/NH-B | <1.0 × 101 | <1.0 × 101 | <1.0 × 101 | |
| Injection of water with biopolymer (biotic control) | RW/SG | 1.0 × 102 | 1.0 × 102 | 1.0 × 103 |
| RW/NH-A | 1.0 × 103 | <1.0 × 101 | <1.0 × 101 | |
| RW/NH-B | 1.0 × 103 | <1.0 × 101 | <1.0 × 101 | |
| Injection of water with biopolymer plus biocide (biotic system) | RW/SG/B | <1.0 × 101 | 1.0 × 101 | 1.0 × 101 |
| RW/NH-A/B | <1.0 × 101 | <1.0 × 101 | <1.0 × 101 | |
| RW/NH-B/B | <1.0 × 101 | <1.0 × 101 | <1.0 × 101 | |
| Injection water | RW | 1.0 × 103 | 1.0 × 102 | 1.0 × 101 |
| Biological System | ||||||
|---|---|---|---|---|---|---|
| Negative Control (Injection Water) | Injection Water (Bacteria) with 25 ppm NPs | Injection Water (Bacteria) with 50 ppm NPs | Injection Water (Bacteria) with 78 ppm NPs | Injection Water (Bacteria) with 100 ppm NPs | Injection Water (Bacteria) with 150 ppm NPs | Injection Water (Bacteria) with 100 ppm Biocide |
| Culture media pre-exposed to endogenous bacteria from the injection water | ||||||
 |  |  |  |  |  |  |
| Culture media exposed to endogenous bacteria from the injection water | ||||||
 |  |  |  |  |  |  |
| Bacterial viability | ||||||
| + | + | + | + | + | + | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, R.H.; Corredor, L.M.; Llanos, S.; Rodríguez, Z.P.; Burgos, I.; Niño, J.A.; Idrobo, E.A.; Romero Bohórquez, A.R.; Zapata Acosta, K.; Franco, C.A.; et al. Evaluation of the Thermal, Chemical, Mechanical, and Microbial Stability of New Nanohybrids Based on Carboxymethyl-Scleroglucan and Silica Nanoparticles for EOR Applications. Nanomaterials 2024, 14, 676. https://doi.org/10.3390/nano14080676
Castro RH, Corredor LM, Llanos S, Rodríguez ZP, Burgos I, Niño JA, Idrobo EA, Romero Bohórquez AR, Zapata Acosta K, Franco CA, et al. Evaluation of the Thermal, Chemical, Mechanical, and Microbial Stability of New Nanohybrids Based on Carboxymethyl-Scleroglucan and Silica Nanoparticles for EOR Applications. Nanomaterials. 2024; 14(8):676. https://doi.org/10.3390/nano14080676
Chicago/Turabian StyleCastro, Rubén H., Laura M. Corredor, Sebastián Llanos, Zully P. Rodríguez, Isidro Burgos, Jhorman A. Niño, Eduardo A. Idrobo, Arnold R. Romero Bohórquez, Karol Zapata Acosta, Camilo A. Franco, and et al. 2024. "Evaluation of the Thermal, Chemical, Mechanical, and Microbial Stability of New Nanohybrids Based on Carboxymethyl-Scleroglucan and Silica Nanoparticles for EOR Applications" Nanomaterials 14, no. 8: 676. https://doi.org/10.3390/nano14080676
APA StyleCastro, R. H., Corredor, L. M., Llanos, S., Rodríguez, Z. P., Burgos, I., Niño, J. A., Idrobo, E. A., Romero Bohórquez, A. R., Zapata Acosta, K., Franco, C. A., & Cortés, F. B. (2024). Evaluation of the Thermal, Chemical, Mechanical, and Microbial Stability of New Nanohybrids Based on Carboxymethyl-Scleroglucan and Silica Nanoparticles for EOR Applications. Nanomaterials, 14(8), 676. https://doi.org/10.3390/nano14080676