One-Pot Solvothermal Synthesis of Highly Emissive, Sodium-Codoped, LaF3 and BaLaF5 Core-Shell Upconverting Nanocrystals
Abstract
:1. Introduction
2. Results and Discussion
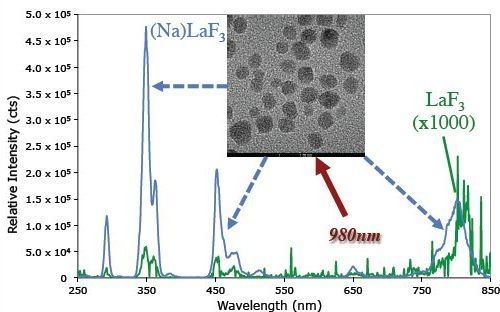
2.1. UCNC Materials and Emission Profiles


2.2. Crystal Structure and Emissive Ion Environment

| Conc. Na | Conc. Ba | Conc. La | Conc. Tm | Conc. Yb | Conc. Ratio | |
| mol%/L | mol%/L | mol%/L | mol%/L | mol%/L | (Yb/Tm): La | |
| (Na)LaF3 Core | 22.15 | ND | 62.11 | 0.38 | 15.36 | 0.26 |
| (Na)LaF3 Core@Shell | 19.02 | ND | 67.09 | 0.34 | 13.55 | 0.12 |
| Ba(Na)LaF5 Core | 25.68 | 31.58 | 33.99 | 0.21 | 8.54 | 0.25 |
| Ba(Na)LaF5 Core@Shell | 12.82 | 25.02 | 55.59 | 0.18 | 6.38 | 0.21 |
| Na:RE (%) | Ba:RE (%) | La:RE (%) | Tm:RE (%) | Yb:RE (%) | Sum mol% RE | |
| (Na)LaF3 Core | 28.45 | ND | 79.78 | 0.49 | 19.73 | 77.85 |
| (Na)LaF3 Core@Shell | 23.48 | ND | 82.85 | 0.42 | 16.73 | 80.98 |
| Ba(Na)LaF5 Core | 60.08 | 73.89 | 79.53 | 0.49 | 19.98 | 42.74 |
| Ba(Na)LaF5 Core@Shell | 20.63 | 40.26 | 89.45 | 0.29 | 10.27 | 62.15 |
| Regions (fitted): | (Na)LaF3 | LaF3 | Ba(Na)LaF5 | BaLaF5 |
|---|---|---|---|---|
| Na 1s | 1071.25 eV (97.35%) | ND | 1071.25 eV | ND |
| Na 1s interstitial | 1069.2 eV (2.65%) | ND | ND | ND |
| La 3d3/2 | 852.94 eV (38.64%) | 853.21 eV | 852.87 eV | 852.87 eV |
| La 3d3/2 interstitial | 851.44 eV (1.23%) | ND | ND | ND |
| La 3d5/2 | 836.134 eV (58.71%) | 836.38 eV | 836.04 eV | 836.12 eV |
| La 3d5/2 interstitial | 834.16 eV (1.43%) | ND | ND | ND |
| Ba 3d3/2 | ND | ND | 794.94 eV (38.94%) | 795.07 eV |
| Ba 3d3/2 interstitial | ND | ND | 793.77 eV (1.55%) | ND |
| Ba 3d5/2 | ND | ND | 779.64 eV (58.75%) | 779.77 eV |
| Ba 3d5/2 interstitial | ND | ND | 778.21 eV (0.77%) | ND |

2.3. Core@Shell Compositional Analysis
| XPS Survey Scan | Na (%) | Ba (%) | La (%) | Yb/Tm (%) | F (%) | Yb/Tm: La |
|---|---|---|---|---|---|---|
| (Na)LaF3 Core | 3.43 | ND | 13.9 | 1.37 | 81.31 | 0.10 |
| (Na)LaF3 Core@Shell | 2.14 | ND | 17.32 | 0.61 | 79.92 | 0.04 |
| Ba(Na)LaF5 Core | 4.94 | 11.56 | 6.61 | 0.65 | 76.24 | 0.10 |
| Ba(Na)LaF5 Core@Shell | 0.0 | 8.56 | 13.58 | 0.69 | 77.17 | 0.05 |
2.4. Emission Intensity versus Power (IvP) Dependence Studies


| (a) | (b) | |||||||
| (Na)LaF3 UCNC | Transition | slope | slope sat. | (Na)LaF3 core@shell | Transition | slope | slope sat. | |
| 292 | 1I6 → 3H6 | 2.16 | 1.25 | 290 | 1I6 → 3H6 | 3.02 | 1.44 | |
| 349 | 1I6 → 3F4 | 2.25 | 1.24 | 346 | 1I6 → 3F4 | 3.10 | 1.2 | |
| 362 | 1D2 → 3H6 | 1.67 | NA | 360 | 1D2 → 3H6 | 2.36 | 1.18 | |
| 452 | 1D2 → 3F4 | 1.65 | NA | 451 | 1D2 → 3F4 | 2.36 | 1.16 | |
| 480 | 1G4 → 3H6 | 1.57 | NA | 479 | 1G4 → 3H6 | Sat. | 1.42 | |
| 514 | 1D2 → 3H5 | 1.83 | NA | 510 | 1D2 → 3H5 | 2.12 | 1.41 | |
| 650 | 1G4 → 3F4 | 1.61 | NA | 647 | 1G4 → 3F4 | 1.63 | 1.37 | |
| 776 | 3F3 → 3H6 | Sat. | 1.09 | 743 | 3F3 → 3H6 | 1.70 | 1.41 | |
| 788 | 3H4 → 3H6 | Sat. | 1.16 | 787 | 3H4 → 3H6 | Sat. | 0.88 | |
| 801 | 3H4 → 3H6 | Sat. | 1.04 | 803 | 3H4 → 3H6 | Sat. | 0.88 | |
| (c) | (d) | |||||||
| Ba(Na)LaF5 UCNC | Transition | slope | slope sat. | Ba(Na)LaF5 core@shell | Transition | slope | slope sat. | |
| 292 | 1I6 → 3H6 | 2.37 | 1.38 | 292 | 1I6 → 3H6 | 2.21 | NA | |
| 349 | 1I6 → 3F4 | 2.38 | 1.36 | 349 | 1I6 → 3F4 | 2.28 | 1.38 | |
| 362 | 1D2 → 3H6 | 1.69 | NA | 362 | 1D2 → 3H6 | 1.71 | NA | |
| 452 | 1D2 → 3F4 | 1.69 | NA | 452 | 1D2 → 3F4 | 1.65 | NA | |
| 463 | 1D2 → 3F4 | 1.63 | NA | 463 | 1D2 → 3F4 | 1.77 | NA | |
| 480 | 1G4 → 3H6 | 1.49 | NA | 480 | 1G4 → 3H6 | 1.60 | NA | |
| 515 | 1D2 → 3H5 | 1.70 | NA | 515 | 1D2 → 3H5 | 1.91 | NA | |
| 650 | 1G4 → 3F4 | 1.32 | NA | 650 | 1G4 → 3F4 | 1.63 | NA | |
| 772 | 3F3 → 3H6 | 1.54 | NA | 772 | 3F3 → 3H6 | 1.50 | NA | |
| 788 | 3H4 → 3H6 | 1.24 | NA | 788 | 3H4 → 3H6 | 1.27 | NA | |
3. Experimental Section
3.1. Synthesis
3.1.1. Materials
3.1.2. Na-Doped LaF3 Synthesis
3.1.3. Na-Doped BaLaF5 Synthesis
3.1.4. (Na)LaF3@(Na)LaF3 and Ba(Na)LaF5@Ba(Na)LaF5 Core/Shell Syntheses
3.2. Characterization
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Conflicts of Interest
References
- Xu, C.F.; Ma, M.; Yang, L.W.; Zeng, S.J.; Yang, Q.B. Lanthanide doping-facilitated growth of ultrasmall monodisperse Ba2LaF7 nanocrystals with excellent photoluminescence. J. Colloid Interface Sci. 2012, 368, 49–55. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X. Upconversion multicolor fine-tuning: Visible to near-infrared emission from lanthanide-doped NaYF4 nanoparticles. J. Am. Chem. Soc. 2008, 130, 5642–5643. [Google Scholar] [CrossRef]
- Wang, F.; Liu, X. Recent advances in the chemistry of lanthanide-doped upconversion nanocrystals. Chem. Soc. Rev. 2009, 38, 976–989. [Google Scholar] [CrossRef]
- Yi, G.-S.; Chow, G.-M. Colloidal LaF3:Yb,Er, LaF3:Yb,Ho and LaF3:Yb,Tm nanocrystals with multicolor upconversion fluorescence. J. Mater. Chem. 2005, 15, 4460–4464. [Google Scholar] [CrossRef]
- Chen, G.; Shen, J.; Ohulchanskyy, T.Y.; Patel, N.J.; Kutikov, A.; Li, Z.; Song, J.; Pandey, R.K.; Ågren, H.; Prasad, P.N. (α-NaYbF4:Tm3+)/CaF2 core/shell nanoparticles with efficient near-infrared to near-infrared upconversion for high-contrast deep tissue bioimaging. ACS Nano 2012, 6, 8280–8287. [Google Scholar] [CrossRef]
- Dong, C.; Pichaandi, J.; Regier, T.; van Veggel, F.C.J.M. Nonstatistical dopant distribution of Ln3+-doped NaGdF4 nanoparticles. J. Phys. Chem. C 2011, 115, 15950–15958. [Google Scholar]
- Chen, G.; Ohulchanskyy, T.Y.; Kumar, R.; Ågren, H.; Prasad, P.N. Ultrasmall monodisperse NaYF4:Yb3+/Tm3+ nanocrystals with enhanced near-infrared to near-infrared upconversion photoluminescence. ACS Nano 2010, 4, 3163–3168. [Google Scholar] [CrossRef]
- Pichaandi, J.; Boyer, J.-C.; Delaney, K.R.; van Veggel, F.C.J.M. Two-photon upconversion laser (scanning and wide-field) microscopy using Ln3+-doped NaYF4 upconverting nanocrystals: A critical evaluation of their performance and potential in bioimaging. J. Phys. Chem. C 2011, 115, 19054–19064. [Google Scholar]
- Chatterjee, D.K.; Fong, L.S.; Zhang, Y. Nanoparticles in photodynamic therapy: An emerging paradigm. Adv. Drug Deliv. Rev. 2008, 60, 1627–1637. [Google Scholar] [CrossRef]
- Yang, Y.; Velmurugan, B.; Liu, X.; Xing, B. NIR photoresponsive crosslinked upconverting nanocarriers toward selective intracellular drug release. Small 2013, 9, 2937–2944. [Google Scholar] [CrossRef]
- Yang, Y.; Shao, Q.; Deng, R.; Wang, C.; Teng, X.; Cheng, K.; Cheng, Z.; Huang, L.; Liu, Z.; Liu, X.; et al. In vitro and in vivo uncaging and bioluminescence imaging by using photocaged upconversion nanoparticles. Angew. Chem. 2012, 51, 3125–3129. [Google Scholar] [CrossRef]
- Auzel, F. Upconversion and anti-stokes processes with f and d ions in solids. Chem. Rev. 2004, 104, 139–173. [Google Scholar] [CrossRef]
- Suyver, J.F.; Grimm, J.; van Veen, M.K.; Biner, D.; Krämer, K.W.; Güdel, H.U. Upconversion spectroscopy and properties of NaYF4 doped with Er3+, Tm3+ and/or Yb3+. J. Lumin. 2006, 117, 1–12. [Google Scholar] [CrossRef]
- Huang, T.C.; Hsieh, W.F. Er-Yb codoped ferroelectrics for controlling visible upconversion emissions. J. Fluoresc. 2009, 19, 511–516. [Google Scholar] [CrossRef]
- Mai, H.-X.; Zhang, Y.-W.; Sun, L.-D.; Yan, C.-H. Highly efficient multicolor up-conversion emissions and their mechanisms of monodisperse NaYF4:Yb,Er core and core/shell-structured nanocrystals. J. Phys.Chem. C 2007, 111, 13721–13729. [Google Scholar] [CrossRef]
- Wang, F.; Deng, R.; Wang, J.; Wang, Q.; Han, Y.; Zhu, H.; Chen, X.; Liu, X. Tuning upconversion through energy migration in core–shell nanoparticles. Nat. Mater. 2011, 10, 968–973. [Google Scholar] [CrossRef]
- Zheng, K.; Qin, W.; Wang, G.; Wei, G.; Zhang, D.; Wang, L.; Kim, R.; Liu, N.; Ding, F.; Xue, X. Upconversion luminescence properties of Yb3+, Gd3+ and Tm3+ co-doped NaYF4 microcrystals synthesized by the hydrothermal method. J. Nanosci. Nanotechnol. 2010, 10, 1920–1923. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, K.; Liu, X.; Dohnalová, K.I.; Gregorkiewicz, T.; Kong, X.; Aalders, M.C.G.; Buma, W.J.; Zhang, H. Critical shell thickness of core/shell upconversion luminescence nanoplatform for FRET application. J. Phys. Chem. Lett. 2011, 2, 2083–2088. [Google Scholar] [CrossRef]
- Cheng, Q.; Sui, J.; Cai, W. Enhanced upconversion emission in Yb3+ and Er3+ codoped NaGdF4 nanocrystals by introducing Li+ ions. Nanoscale 2012, 4, 779–784. [Google Scholar] [CrossRef]
- Huang, Q.; Yu, J.; Ma, E.; Lin, K. Synthesis and characterization of highly efficient near-infrared upconversion Sc3+/Er3+/Yb3+ tridoped NaYF4. J. Phys. Chem. C 2010, 114, 4719–4724. [Google Scholar] [CrossRef]
- Chen, D.; Yu, Y.; Huang, F.; Wang, Y. Phase transition from hexagonal LnF3 (Ln = La, Ce, Pr) to cubic Ln0.8M0.2F2.8 (M = Ca, Sr, Ba) nanocrystals with enhanced upconversion induced by alkaline-earth doping. Chem. Commun. 2011, 47, 2601–2603. [Google Scholar] [CrossRef]
- Chen, G.Y.; Liu, H.C.; Somesfalean, G.; Sheng, Y.Q.; Liang, H.J.; Zhang, Z.G.; Sun, Q.; Wang, F.P. Enhancement of the upconversion radiation in Y2O3:Er3+ nanocrystals by codoping with Li+ ions. Appl. Phys. Lett. 2008, 92, 96–100. [Google Scholar]
- Wang, C.; Tao, H.; Cheng, L.; Liu, Z. Near-infrared light induced in vivo photodynamic therapy of cancer based on upconversion nanoparticles. Biomaterials 2011, 32, 6145–6154. [Google Scholar]
- Xiong, L.; Yang, T.; Yang, Y.; Xu, C.; Li, F. Long-term in vivo biodistribution imaging and toxicity of polyacrylic acid-coated upconversion nanophosphors. Biomaterials 2010, 31, 7078–7085. [Google Scholar] [CrossRef]
- Jalil, R.A.; Zhang, Y. Biocompatibility of silica coated NaYF4 upconversion fluorescent nanocrystals. Biomaterials 2008, 29, 4122–4128. [Google Scholar] [CrossRef]
- Li, C.; Lin, J. Rare earth fluoride nano-/microcrystals: Synthesis, surface modification and application. J. Mater. Chem. 2010, 20, 6831–6847. [Google Scholar] [CrossRef]
- Hu, H.; Xiong, L.; Zhou, J.; Li, F.; Cao, T.; Huang, C. Multimodal-luminescence core-shell nanocomposites for targeted imaging of tumor cells. Chemistry 2009, 15, 3577–3584. [Google Scholar] [CrossRef]
- Wang, L.; Yan, R.; Huo, Z.; Wang, L.; Zeng, J.; Bao, J.; Wang, X.; Peng, Q.; Li, Y. Fluorescence resonant energy transfer biosensor based on upconversion-luminescent nanoparticles. Angew. Chem. 2005, 117, 6208–6211. [Google Scholar] [CrossRef]
- Mahalingam, V.; Vetrone, F.; Naccache, R.; Speghini, A.; Capobianco, J.A. Colloidal Tm3+/Yb3+-doped LiYF4 nanocrystals: Multiple luminescence spanning the UV to NIR regions via low-energy excitation. Adv. Mater. 2009, 21, 4025–4028. [Google Scholar] [CrossRef]
- Wang, G.; Qin, W.; Wang, L.; Wei, G.; Zhu, P.; Kim, R. Intense ultraviolet upconversion luminescence from hexagonal NaYF4:Yb3+/Tm3+ microcrystals. Opt. Express 2008, 16, 11907–11914. [Google Scholar] [CrossRef]
- Balakrishnaiah, R.; Kim, D.W.; Yi, S.S.; Jang, K.; Lee, H.S.; Ahn, C.W.; Jeong, J.H. Infrared to ultraviolet frequency upconversion studies of Tm3+ ions in a YbLiF4 crystal. J. Korean Phys. Soc. 2008, 53, 791–795. [Google Scholar] [CrossRef]
- Shan, J.; Ju, Y. A single-step synthesis and the kinetic mechanism for monodisperse and hexagonal-phase NaYF4:Yb,Er upconversion nanophosphors. Nanotechnology 2009, 20, 275603:1–275603:13. [Google Scholar]
- Chen, X.Y.; Zhuang, H.Z.; Liu, G.K.; Li, S.; Niedbala, R.S. Confinement on energy transfer between luminescent centers in nanocrystals. J. Appl. Phys. 2003, 94, 5559–5565. [Google Scholar] [CrossRef]
- Wang, Y.; Qin Wei, P.; Di, W.-H.; Zhang Ji, S.; Cao, C.-Y. Infrared-to-visible and infrared-to-violet upconversion fluorescence of rare earth doped LaF3 nanocrystals. Chin. Phys. B 2008, 17, 3300–3305. [Google Scholar] [CrossRef]
- Yi, G.-S.; Chow, G.-M. Water-soluble NaYF4:Yb,Er(Tm)/NaYF4/polymer core/shell/shell nanoparticles with significant enhancement of upconversion fluorescence. Chem. Mater. 2007, 19, 341–343. [Google Scholar] [CrossRef]
- Liu, Y.; Tu, D.; Zhu, H.; Li, R.; Luo, W.; Chen, X. A strategy to achieve efficient dual-mode luminescence of Eu3+ in lanthanides doped multifunctional NaGdF4 nanocrystals. Adv. Mater. 2010, 22, 3266–3271. [Google Scholar] [CrossRef]
- Chen, G.; Yang, C.; Prasad, P.N. Nanophotonics and nanochemistry: Controlling the excitation dynamics for frequency up- and down-conversion in lanthanide-doped nanoparticles. Acc. Chem. Res. 2013, 46, 1474–1486. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, Q.-M. Synthesis and photoluminescent properties of α-NaYF4:Nd/α- NaYF4 core/shell nanostructure with enhanced near infrared (NIR) emission. Mater. Lett. 2009, 63, 376–378. [Google Scholar] [CrossRef]
- Qu, Y.; Li, M.; Zhang, L.; Zhao, L. Preparation and characterization of upconversion luminescent LaF3:Yb3+, Er3+/LaF3 core/shell nanocrystals. Appl. Surf. Sci. 2011, 258, 34–37. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Y.; Yang, K.; Zhang, X.; Song, Y.; Wang, C.H. Enhanced upconverted photoluminescence in Er3+ and Yb3+ codoped ZnO nanocrystals with and without Li+ ions. Opt. Commun. 2008, 281, 5448–5452. [Google Scholar] [CrossRef]
- Chen, G.Y.; Liu, H.C.; Liang, H.J.; Somesfalean, G.; Zhang, Z.G. Enhanced multiphoton ultraviolet and blue upconversion emissions in Y2O3:Er3+ nanocrystals by codoping with Li+ ions. Solid State Commun. 2008, 148, 96–100. [Google Scholar] [CrossRef]
- Awan, S.U.; Hasanain, S.K.; Bertino, M.F.; Jaffari, G.H. Ferromagnetism in Li doped ZnO nanoparticles: The role of interstitial Li. J. Appl. Phys. 2012, 112, 103924:1–103924:9. [Google Scholar]
- Cates, E.L.; Wilkinson, A.P.; Kim, J.-H. Delineating mechanisms of upconversion enhancement by Li+ codoping in Y2SiO5:Pr3+. J. Phys. Chem. C 2012, 116, 12772–12778. [Google Scholar] [CrossRef]
- Baikie, T.; Ng, G.M.; Madhavi, S.; Pramana, S.S.; Blake, K.; Elcombe, M.; White, T.J. The crystal chemistry of the alkaline-earth apatites A10(PO4)6CuxOy(H)z (A = Ca, Sr and Ba). Dalton Trans. 2009, 34, 6722–6726. [Google Scholar]
- Haase, M.; Schafer, H. Upconverting nanoparticles. Angew. Chem. 2011, 50, 5808–5829. [Google Scholar] [CrossRef]
- Mai, H.-X.; Zhang, Y.-W.; Sun, L.-D.; Yan, C.-H. Size- and phase-controlled synthesis of monodisperse NaYF4:Yb,Er nanocrystals from a unique delayed nucleation pathway monitored with upconversion spectroscopy. J. Phys. Chem. C 2007, 111, 13730–13739. [Google Scholar] [CrossRef]
- Pollnau, M.; Gamelin, D.R.; Lüthi, S.R.; Güdel, H.U.; Hehlen, M.P. Power dependence of upconversion luminescence in lanthanide and transition-metal-ion systems. Phys. Rev. B 2000, 61, 3337–3346. [Google Scholar] [CrossRef]
- Suyver, J.; Aebischer, A.; García-Revilla, S.; Gerner, P.; Güdel, H. Anomalous power dependence of sensitized upconversion luminescence. Phys. Rev. B 2005, 71, 125123:1–125123:9. [Google Scholar]
- Renero-Lecuna, C.; Martín-Rodríguez, R.; Valiente, R.; González, J.; Rodríguez, F.; Krämer, K.W.; Güdel, H.U. Origin of the high upconversion green luminescence efficiency in β-NaYF4:2% Er3+, 20% Yb3+. Chem. Mater. 2011, 23, 3442–3448. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Stecher, J.T.; Rohlfing, A.B.; Therien, M.J. One-Pot Solvothermal Synthesis of Highly Emissive, Sodium-Codoped, LaF3 and BaLaF5 Core-Shell Upconverting Nanocrystals. Nanomaterials 2014, 4, 69-86. https://doi.org/10.3390/nano4010069
Stecher JT, Rohlfing AB, Therien MJ. One-Pot Solvothermal Synthesis of Highly Emissive, Sodium-Codoped, LaF3 and BaLaF5 Core-Shell Upconverting Nanocrystals. Nanomaterials. 2014; 4(1):69-86. https://doi.org/10.3390/nano4010069
Chicago/Turabian StyleStecher, Joshua T., Anne B. Rohlfing, and Michael J. Therien. 2014. "One-Pot Solvothermal Synthesis of Highly Emissive, Sodium-Codoped, LaF3 and BaLaF5 Core-Shell Upconverting Nanocrystals" Nanomaterials 4, no. 1: 69-86. https://doi.org/10.3390/nano4010069
APA StyleStecher, J. T., Rohlfing, A. B., & Therien, M. J. (2014). One-Pot Solvothermal Synthesis of Highly Emissive, Sodium-Codoped, LaF3 and BaLaF5 Core-Shell Upconverting Nanocrystals. Nanomaterials, 4(1), 69-86. https://doi.org/10.3390/nano4010069