Work Function Engineering of Graphene
Abstract
:1. Scope of the Review

2. Graphene: A Unique Carbon Allotrope


3. Graphene: A Two Dimensional Form of Carbon with Unusual Band Structure and Characteristics

- (1)
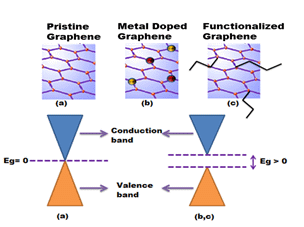
- Graphene is a zero-band gap perfect 2D semi-conductor with a tiny overlap between valence and conduction bands [6];
- (2)
- (3)
- Graphene shows a strong ambipolar electric field effect with charge carrier concentrations up to 1013 cm2 and room temperature mobility of ~10,000 cm2·s−1 [6];
- (4)
- (5)
- Graphene displays high thermal conductivity with a value of ~5000 Wmk−1 for a single layer sheet at room temperature [19];
- (6)
- Graphene exhibits high optical transparency with absorption of ~2.3% towards visible light [20];
- (7)
- Graphene is incredibly strong, mechanically (tensile strength of ~130 GPa, for a defect free single layer, Young’s modulus of 1 Tpa, third order elastic stiffness ≈2 Tpa); while remaining highly flexible and very light (0.77 mg m−2) and possesses a very high specific surface area (~2630 m2·g−1) [21].
4. Graphene: Potential Applications and Importance of Work Function
5. Graphene Synthesis: Relationship to Applications

| Method | Precursor | Electronic quality | Advantage | Disadvantage | Commercialization | References |
|---|---|---|---|---|---|---|
| Mechanical Exfoliation | Graphite | High | Inexpensive and time saving method | Flakes randomly distributed, poor yield | Not scalable for commercialization | [76] |
| Arc discharge method | Graphite | High | Applicable to obtain Boron or nitrogen doped graphene | Cannot obtain pure graphene | Not scalable | [78] |
| Wet chemical synthesis such as Hummer, Brodie | Graphite | High | Transparent conductive film, useful to synthesis graphene based composites | Presence of oxygen impurities are not suitable for most of the electronic applications | Can be obtained in lab but not good enough for commercialization | [92] |
| Chemical vapour deposition | Hydrocarbons | High | Promising method that has all the above mentioned advantages | Transfer of graphene films deteriorates graphene quality and causes wrinkle formation | Possible | [80,82] |
| Solvothermal synthesis | Ethanol | Not available | Cheap and easily available precursor | Popcorn effect arises due to nucleation of sheets | Scalable | [96] |
| Epitaxial growth on metals | Ultrathin graphitic film | High | Single to multi layer graphene sheets can be obtained | Requires high temperature, expensive and difficult transfer process | Not feasible | [97] |
6. Work Function and Tuning of the Work Function of Graphene
6.1. Effect of Oxygen Functionalities on the Work Function of Graphene



6.2. Work Function Engineering by Reduction of Graphene Oxide


6.3. Work Function Engineering of GO Using Functionalization and Self-Assembled Monolayer

6.4. Work Function Engineering Using Self-Assembled Monolayer (SAM) and Layer by Layer Technique

6.5. Work Function Engineering of Graphene Using Noble Metal Doping
6.6. Work Function Engineering on Graphene Based Gold (Au) Composite




6.7. Work Function Engineering on Graphene Based Silver (Ag) Composite
6.8. Work Function Engineering for Graphene Based Platinum (Pt) Composite
| S.No. | Modified Graphene | Method | Precursor | WF (eV) | Improved property | Application | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Graphene-ZnO-Au | Hydrothermal | Zn Acetate , HAuCl4 | Value not given | Efficiency = 3.5–4.5 fold more than ZnO | Removing pollutant (nitrobenzene) | [157] |
| 2 | Various Au dopants on graphene | Chemical doping | AuBr3, Au2S, Au(OH)3, AuCl3 | 5, 4.8, 4.6, 4.9 as doped and 4.5, 4.4, 4.55, 4.3 eV as annealed (w.r.t. the precursor) | Multiuse of graphene due to tunable WF property | Energy conversion devices and sensors | [159] |
| 3 | Au/graphene | Chemical doping | AuCl3 | Increase by 0.5 eV with increase in doping time | Tunable WF property | Optoelectronic devices | [60] |
| 4 | Ag/graphene | Photochemical silver functionalization | AgNO3 | Value not given | Suppressed e-h recombination | Efficient removal of hazardous materials | [161] |
| 5 | Au/Ag/Pt-graphene | Graphene adsorption metal substrate | Au, Ag, Pt substrates were used | 5.54–4.74, 4.92–4.24, 6.13–4.8 eV | Multipurpose modified graphene | Energy conversion devices | [168] |
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Hazen, R.M.; Downs, R.T.; Jones, A. Carbon mineralogy and crystal chemistry. Rev. Mineral. Geochem. 2013, 75, 7–46. [Google Scholar] [CrossRef]
- Oganov, A.R.; Hemley, R.J.; Hazen, R.M. Structure, bonding and mineralogy of carbon at extreme conditions. Rev. Mineral. Geochem. 2013, 75, 47–77. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Biswas, K.; Subrahmanyam, K.S.; Govindaraj, A. Graphene, the new nanocarbon. J. Mater. Chem. 2009, 19, 2457–2469. [Google Scholar]
- Katsnelson, M.I. Graphene: Carbon in two dimensions. Mater. Today 2007, 10, 20–27. [Google Scholar] [CrossRef]
- Katsnelson, M.I.; Novoselov, K.S. Graphene: New bridge between condensed matter physics and quantum electrodynamics. Solid State Commun. 2007, 143, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Ando, T. The electronic properties of graphene and carbon nanotubes. NPG Asia Mater. 2009, 1, 17–21. [Google Scholar] [CrossRef]
- Wallace, P.R. The band theory of graphite. Phys. Rev. 1947, 71, 622–634. [Google Scholar] [CrossRef]
- Ohta, T.; Bostwick, A.; Seyller, T. Controlling the electronic structure of bilayer graphene. Science 2006, 313, 951–954. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Sun, Z.; Hasan, T. Graphene photonics and optoelectronics. Nat. Photonics 2010, 4, 611–622. [Google Scholar] [CrossRef]
- Nardecchia, S.; Monte, F.; Ferrer, M. Three dimensional macroporous architectures and aerogels built of carbon nanotubes and/or graphene: Synthesis and applications. Chem. Soc. Rev. 2013, 42, 794–830. [Google Scholar] [CrossRef]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Avouris, P.; Dimitrakopoulos, C. Graphene: Synthesis and applications. Mater. Today 2012, 15, 86–97. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.W.; Stormer, H.L.; Kim, P. Experimental observation of the quantum hall effect and Berry’s phase in graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef]
- Novoselov, K.S.; McCann, E.; Morozov, S.V.; Falko, V.I.; Katsnelson, M.I.; Zeitler, U.; Jiang, D.; Schedin, F.; Geim, A.K. Unconventional quantum hall effect and Berry’s phase of 2pi in bilayer graphene. Nat. Phys. 2006, 2, 177–180. [Google Scholar]
- Novoselov, K.S.; Jiang, Z.; Zhang, Y.; Morozov, S.V.; Stormer, H.L.; Zeitler, U.; Maan, J.C.; Boebinger, G.S.; Kim, P.; Geim, A.K. Room-temperature quantum hall effect in graphene. Science 2007, 315, 1379. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two dimensional gas of massless Dirac fermion in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.Y.; Gweon, G.H.; Graf, J.; Fedorov, A.V. First direct observation of Dirac fermions in graphite. Nat. Phys. 2006, 2, 595–599. [Google Scholar] [CrossRef]
- Chen, S.; Wu, Q.; Mishra, C. New graphene-related materials on the horizon. Nat. Mater. 2012, 11, 203–207. [Google Scholar] [CrossRef]
- Nair, R.R.; Blake, P.; Grigorenko, A.N. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of mono-layer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Du, W.; Jiang, X.; Zhub, L. From graphite to graphene: Direct liquid phase exfoliation of graphite to produce single and few layered pristine graphene. J. Mater. Chem. A 2013, 1, 10592–10606. [Google Scholar] [CrossRef]
- Krishnan, D.; Kim, F.; Luo, J. Energetic graphene oxide: Challenges and opportunities. Nano Today 2012, 7, 137–152. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, Z.; Chen, Z. Graphene-related nanomaterials: Tuning properties by functionalisation. Nanoscale 2013, 5, 4541–4583. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W. Grpahene and graphene oxide: Synthesis, properties and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar]
- Allen, J.M.; Tung, C.V.; Kaner, R. Honeycomb carbon: A review on graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–199. [Google Scholar] [CrossRef]
- Das Sarma, S.; Geim, A.; Kim, P.; MacDonald, A. Exploring graphene—Recent research advances—Foreword. Solid State Commun. 2007, 143, 1–2. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Q.; Shi, G. Graphene based new energy materials. Energy Environ. Sci. 2011, 4, 1113–1132. [Google Scholar] [CrossRef]
- Chen, D.; Tanga, L.; Li, J. Graphene based material in electrochemistry. Chem. Soc. Rev. 2010, 39, 3157–3180. [Google Scholar] [CrossRef]
- Brownson, D.; Kampouris, K.D. Graphene electrochemistry: Fundamental concepts through to prominent applications. Chem. Soc. Rev. 2012, 41, 6944–6976. [Google Scholar] [CrossRef]
- Compton, C.O.; Nguyen, S. Graphene oxide, highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef]
- Cui, S.; Mao, S.; Lu, G.; Chen, J. Graphene coupled with nanocrystals: Opportunities and challenges for energy and sensing application. J. Phys. Chem. Lett. 2013, 4, 2441–2454. [Google Scholar] [CrossRef]
- Weiss, N.; Zhou, H.; Liao, L.; Liu, Y.; Jiang, S. An emerging electronic material. Adv. Mater. 2012, 24, 5782–5825. [Google Scholar] [CrossRef]
- Wasse, J.K.; Kaner, R.B. Exploring the synthesis and application of graphene. Mater. Today 2010, 13, 52–59. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Hoon, K.N.H.; Lee, J. Chemical functionalization of graphene and its applications. Prog. Mater. Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Yang, K.; Feng, L.; Hong, H.; Cai, W.; Liu, Z. Preparation and functionalization of graphene nanocomposites for biomedical applications. Nat. Protoc. 2013, 8, 2392–2403. [Google Scholar] [CrossRef]
- Craciun, M.F.; Khrapach, I.; Barnes, M.D.; Russo, S. Properties and applications of chemically functionalized graphene. J. Phys. Condens. Matter 2013, 25, 423201:1–423201:42. [Google Scholar]
- Bunch, J.S.; van der Zande, A.M.; Verbridge, S.S.; Frank, I.W.; Tanenbaum, D.M.; Parpia, J.M.; Craighead, H.G. Electromechanical resonators from graphene sheets. Science 2007, 315, 490–493. [Google Scholar] [CrossRef]
- Staley, N.; Wang, H.; Puls, C.; Forster, J.; Jackson, T.N.; McCarthy, K.; Clouser, B.; Liu, Y. Lithography free fabrication of graphene devices. Appl. Phys. Lett. 2007, 90, 143518:1–143518:3. [Google Scholar]
- Gilje, S.; Han, S.; Wang, K.L.; Kaner, R.B. Graphene: Calling all chemists. Nano Lett. 2007, 7, 3394–3398. [Google Scholar] [CrossRef]
- Moore, G.E. Cramming more components onto integrated circuits. Electronics 1965, 38, 114–117. [Google Scholar]
- Son, Y.W.; Cohen, M.L.; Louie, S.G. Energy gap in graphene nanoribbons. Phys. Rev. Lett. 2006, 97, 216803:1–216803:4. [Google Scholar]
- Wang, X.; Zhi, L.; Mullen, K. Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 2008, 8, 323–327. [Google Scholar] [CrossRef]
- Zhu, Y.; Cai, W.; Piner, R.D.; Velamakanni, A.; Ruoffa, R.S. Transparent self-assembled films of reduced graphene oxide platelets. Appl. Phys. Lett. 2009, 95, 103104:1–103104:3. [Google Scholar]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of solution-processed reduced graphene oxide films as transparent conductors. ACS Nano 2008, 2, 463–470. [Google Scholar] [CrossRef]
- Shin, H.J.; Kim, K.K.; Benayad, A.; Yoon, S.; Park, H. Efficient reduction of graphite oxide by sodium borohydride and its effect on electrical conductance. Adv. Funct. Mater. 2009, 19, 1987–1992. [Google Scholar]
- Watcharotone, S.; Dikin, D.; Stankovich, S. Graphene-silica composite thin films as transparent conductors. Nano Lett. 2007, 7, 1888–1892. [Google Scholar] [CrossRef]
- Robinson, J.T.; Perkins, F.K.; Snow, E.S.; Wei, Z.; Sheehan, P.E. Reduced graphene oxide molecular sensors. Nano Lett. 2008, 8, 3137–3140. [Google Scholar] [CrossRef]
- Wang, D.W.; Li, F.; Zhao, J.; Ren, W.; Chen, Z.G.; Tan, J. Fabrication of graphene/polyaniline composite paper via anodic electropolymerization for high-performance flexible electrode. ACS Nano 2009, 3, 1745–1752. [Google Scholar] [CrossRef]
- Andreas, P.; Humer, M.; Furchi, M.M.; Bachmann, D.; Guider, R.; Fromherz, T.; Mueller, T. CMOS-compatible graphene photodetector covering all optical communication bands. Nat. Photonics 2013, 7, 892–896. [Google Scholar] [CrossRef]
- Zhou1, S.Y.; Gweon1, G.H.; Fedorov, A.V. Substrate induced bandgap opening. Nat. Mater. 2007, 6, 770–775. [Google Scholar] [CrossRef]
- Song, S.M.; Park, J.; Sul, O.J. Determination of Work function of graphene under a metal electrode and its role in contact resistance. Nano Lett. 2012, 12, 3887–3892. [Google Scholar] [CrossRef]
- Xia, F.; Mueller, T.; Mojarad, R.; Avouris, P. Photocurrent Imaging and efficient photon detection in a graphene transistor. Nano Lett. 2009, 9, 1039–1044. [Google Scholar] [CrossRef]
- Eda, G.; Unalan, H.E.; Rupesinghe, N.; Chhowalla, M. Graphene based composite thin films. Appl. Phys. Lett. 2008, 93, 233502:1–233502:3. [Google Scholar]
- Yu, Y.-J.; Zhao, Y.; Ryu, S.; Brus, L.E.; Kim, K.S.; Kim, P. Tuning the graphene work function by electric field effect. Nano Lett. 2009, 9, 3430–3434. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Ang, P.K.; Yang, J. The chemistry of graphene. J. Mater. Chem. 2010, 20, 2277–2289. [Google Scholar]
- Shi, Y.; Kim, K.K.; Reina, A.; Hofmann, M.; Li, L.-J.; Kong, J. Work function engineering of graphene electrode via chemical doping. ACS Nano 2010, 4, 2689–2694. [Google Scholar] [CrossRef]
- Giovannetti, G.; Khomyakov, P.A.; Brocks, G.; Karpan, V.M.; van den Brink, J.; Kelly, P.J. Doping graphene with metal contacts. Phys. Rev. Lett. 2008, 101, 026803:1–026803:4. [Google Scholar]
- Benayad, A.; Shin, H.J.; Park, H.K.; Yoon, S.M. Controlling work function of reduced graphite oxide with Au-ion concentration. Chem. Phys. Lett. 2009, 475, 91–95. [Google Scholar] [CrossRef]
- Wang, B.; Gunther, S.; Wintterlin, J. Peridoicity, work function and reactivity of graphene on Ru(001) from first principles. New J. Phys. 2010, 12, 043041:1–043041:15. [Google Scholar]
- Murata, Y.; Starodub, E.; Kappes, B.B.; Ciobanu, C.V.; Bartelt, N.C.; McCarty, K.F.; Kodambaka, S. Orientation-dependent work function of graphene on Pd(111). Appl. Phys. Lett. 2010, 97, 143114:1–143114:3. [Google Scholar]
- Park, J.; Lee, W.H.; Huh, S.; Sim, S.H.; Kim, S.B.; Cho, K.; Hong, B.H.; Kim, K.S. Work-function engineering of graphene electrodes by self-assembled monolayers for high-performance organic field-effect transistors. J. Phys. Chem. Lett. 2011, 2, 841–845. [Google Scholar] [CrossRef]
- Elias, D.C.; Nair, R.R.; Mohiuddin, T.M.G.; Morozov, S.V.; Blake, P.; Halsall, M.P.; Ferrari, A.C.; Boukhvalov, D.W.; Katsnelson, M.I.; Geim, A.K.; et al. Control of graphene’s properties by reversible hydrogenation: Evidence for graphane. Science 2009, 323, 610–613. [Google Scholar] [CrossRef]
- Cheng, Y.C.; Kaloni, T.P.; Huang, G.S.; Schwingenschlögl, U. Origin of the high p-doping in F intercalated graphene on SiC. Appl. Phys. Lett. 2011, 99, 053117:1–053117:3. [Google Scholar]
- Virojanadara, C.; Watcharinyanon, S.; Zakharov, A.A.; Johansson, L.I. Epitaxial graphene on SiC and Li intercalation. Phys. Rev. B 2010, 82, 205402:1–205402:6. [Google Scholar]
- Premlal, B.; Cranney, M.; Vonau, F.; Aubel, D.; Casterman, D.; De Souza, M.M.; Simon, L. Surface intercalation of gold underneath a graphene monolayer on SiC(0001) studied by scanning tunneling microscopy and spectroscopy. Appl. Phys. Lett. 2009, 94, 263115:1–263115:3. [Google Scholar]
- Khrapach, I.; Withers, F.; Bointon, T.H.; Polyushkin, D.K.; Barnes, W.L.; Russo, S.; Craciun, M.F. Novel highly conductive and transparent graphene-based conductors. Adv. Mater. 2012, 24, 2844–2849. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Tan, P.H.; Liu, J.; Ferrari, A.C. Intercalation of few-layer graphite flakes with FeCl3: Raman determination of fermi level, layer by layer decoupling, and stability. J. Am. Chem. Soc. 2011, 133, 5941–5946. [Google Scholar] [CrossRef]
- McAllister, M.J.; Li, J.; Adamson, D.H.; Schniepp, H.C.; Aksay, I.A. Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem. Mater. 2007, 19, 4396–4406. [Google Scholar] [CrossRef]
- Lotya, M.; Hernandez, Y.; King, P.J. Solvation assisted exfoliation of graphite. Chem. Soc. 2009, 131, 3611–3620. [Google Scholar] [CrossRef]
- Kim, K.S.; Zhao, Y.; Jang, H.; Lee, S.Y. Pattern growth of graphene films for stretchable transparent electrodes. Nature 2009, 457, 706–710. [Google Scholar] [CrossRef]
- Obraztsov, A.N. Chemical vapour deposition: Making graphene on a large scale. Nat. Nano 2009, 4, 212–213. [Google Scholar] [CrossRef]
- Cai, M.; Thorpe, D.; Adamson, D.H.; Schniepp, H.C. Methods of graphite exfoliation. J. Mater. Chem. 2012, 22, 24992–25002. [Google Scholar] [CrossRef]
- Chen, L.; Hernandez, Y.; Feng, X.; Müllen, K. From nanographene and graphene nanoribbons to graphene sheets: Chemical synthesis. Angew. Chem. Int. Ed. 2012, 51, 7640–7654. [Google Scholar]
- Wu, C.; Dong, G.; Guan, L. Production of graphene sheets by a simple helium arc-discharge. Phys. E 2010, 42, 1267–1271. [Google Scholar] [CrossRef]
- Kumar, P. Laser flash synthesis of graphene and its inorganic analogues: An innovative breakthrough with immense promise. RSC Adv. 2013, 3, 11987–12002. [Google Scholar] [CrossRef]
- Liao, C.D.; Lu, Y.Y.; Tamalampudi, S.R.; Cheng, H.C.; Chen, Y.T. Chemical vapor deposition synthesis and raman spectroscopic characterization of large-area graphene sheets. J. Phys. Chem. A 2013, 117, 9454–9461. [Google Scholar] [CrossRef]
- Kuila, T.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Recent advances in the efficient reduction of graphene oxide and its application as energy storage electrode materials. Nanoscale 2013, 5, 52–71. [Google Scholar]
- Li, X.S.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef]
- Lin, Y.M.; Dimitrakopoulos, C.; Jenkins, K.A.; Farmer, D.B.; Chiu, H.-Y.; Grill, A.; Avouris, P. 100-GHz transistors from wafer-scale epitaxial graphene. Science 2010, 327, 662. [Google Scholar] [CrossRef]
- Colson, J.W.; Woll, A.R.; Mukherjee, A.; Spitler, E.L.; Shields, V.B.; Spencer, M.G.; Park, J.; Dichtel, W.R. Oriented 2D. Science 2011, 332, 228–231. [Google Scholar] [CrossRef]
- Gunasinghe, R.N.; Reuven, D.G.; Suggs, K.; Wang, X.-Q. Filled and empty orbital interactions in a planar covalent organic framework on graphene. J. Phys. Chem. Lett. 2012, 3, 3048–3052. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, S.R. Chemical methods for the production of graphene. Nanotechnology 2009, 4, 217–224. [Google Scholar]
- Brodie, C. On the atomic weight of graphite. Phil. Trans. R. Soc. A 1859, 149, 249–259. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offerman, R.E. Preparation of graphite oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Hirata, M.; Gotou, T.; Horiuchi, S.; Fujiwara, M.; Ohba, M. Progress towards monodisperse single-walled carbon nanotubes. Carbon 2004, 42, 2929–2937. [Google Scholar]
- Wei, G.; Lawrence, A.; Li, B. New insights into the structure and reduction of graphite oxide. Nat. Chem. 2009, 1, 403–408. [Google Scholar] [CrossRef]
- Jeong, H.K.; Lee, Y.P.; Jin, M.H.; Kim, E.S.; Bae, J.J.; Lee, Y.H. Thermal stability of graphite oxide. Chem. Phys. Lett. 2009, 470, 255–258. [Google Scholar]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Ambrosi, A.; Bonanni, A.; Sofer, Z.K.; Cross, J.S.; Pumera, M. Electrochemistry at chemically modified graphenes. Chem. Eur. J. 2011, 17, 10763–10770. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef]
- Mao, S.; Pu, H.; Chen, J. Graphene oxide and its reduction: Modeling and experimental progress. RSC Adv. 2012, 2, 2643–2662. [Google Scholar] [CrossRef]
- Choucair, M.; Thordarson, P.; Stride, J.A. Gram-scale production of graphene based on solvothermal synthesis and sonication. Nat. Nano 2009, 4, 30–33. [Google Scholar] [CrossRef]
- Zangwill, A.; Vvedensky, D.D. Novel growth mechanism of epitaxial graphene on metals. Nano Lett. 2011, 11, 2092–2095. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Wu, H. Graphene based electrochemical sensors and biosensors. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Monsur, H.; Hyejin, P.; Sung, D. An electrochemically reduced graphene oxide-based electrochemical immunosensing platform for ultrasensitive antigen detection. Anal. Chem. 2012, 84, 1871–1878. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Engelhard, M. Facile and controllable electrochemical reduction of graphene oxide and its applications. J. Mater. Chem. 2010, 20, 743–748. [Google Scholar] [CrossRef]
- Peng, X.; Tang, F.; Copple, A. Engineering the work function of armchair graphene nanoribbons using strain and functional species: A first principles study. J. Phys. Condens. Matter 2012, 24, 075501:1–075501:10. [Google Scholar]
- Jiang, J.; Krauss, T.D.; Brus, L. Electrostatic force microscopy characterization of trioctylphosphine oxide self-assembled monolayers on graphite. J. Phys. Chem. B 2000, 104, 11936–11941. [Google Scholar] [CrossRef]
- Shin, H.J.; Choi, W.M.; Choi, D.; Han, G.H. Control of electronic structure of graphene by various dopants and their effects on a nanogenerator. J. Am. Chem. Soc. 2010, 132, 15603–15609. [Google Scholar] [CrossRef]
- Nduwimana, A.; Wang, X.-Q. Energy gaps in supramolecular functionalized graphene nanoribbons. ACS Nano 2012, 6, 1011–1017. [Google Scholar] [CrossRef]
- Williams, M.D.; Samarakoon, D.K.; Hessc, D.W.; Wang, X.-Q. Tunable bands in biased multilayer epitaxial grapheme. Nanoscale 2012, 4, 2962–2967. [Google Scholar] [CrossRef]
- Singh, A.K.; Ahmad, M.; Singh, V. Tailoring the electrical properties of graphene layers by molecular doping. ACS Appl. Mater. Interfaces 2013, 5, 5276–5281. [Google Scholar] [CrossRef]
- Lee, J.; Novoselov, K.S.; Shin, H.S. Interaction between metal and graphene: Dependence on the layer number of graphene. ACS Nano 2011, 5, 608–612. [Google Scholar] [CrossRef]
- Wang, X.R.; Li, X.L.; Zhang, L. N-doping of graphene through electrothermal reactions with ammonia. J. Sci. 2009, 324, 768–771. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B. Functionalization of graphene: Covalent and non-covalent approaches. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Peimyoo, N.; Yu, T.; Shang, J.; Cong, C.; Yang, H. Thickness-dependent azobenzene doping in mono- and few-layer graphene. Carbon 2012, 50, 201–208. [Google Scholar] [CrossRef]
- Dong, X.; Long, Q.; Wei, A.; Huang, W. The electrical properties of graphene modified by bromophenyl groups derived from a diazonium compound. Carbon 2012, 50, 1517–1522. [Google Scholar] [CrossRef]
- Yu, Y.J.; Zhao, Y.; Ryu, S.; Brus, L.E.; Kim, K.S. Tuning the graphene work function by electric field effect. Nano Lett. 2009, 10, 3430–3434. [Google Scholar]
- Gui, G.; Li, J.; Zhong, J. Band structure engineering of graphene by strain: First-principles calculations. Phys. Rev. B 2008, 78, 075435:1–075435:3. [Google Scholar]
- Zhang, Z.; Huang, H.; Yang, X.; Zang, L. Tailoring electronic properties of graphene by π–π stacking with aromatic molecules. J. Phys. Chem. Lett. 2011, 2, 2897–2905. [Google Scholar] [CrossRef]
- Singh, A.K.; Iqbal, M.W.; Singh, V.K.; Iqbal, M.Z. Molecular n-doping of chemical vapour deposition. Mater. Chem. 2012, 22, 15168–15174. [Google Scholar]
- Eda, G.; Lin, Y.-Y.; Mattevi, C. Blue photoluminescence from chemically derived graphene oxide. Adv. Mater. 2010, 22, 505–509. [Google Scholar] [CrossRef]
- Matis, B.R.; Burgess, J.S.; Bulat, F.A.; Friedman, A.L.; Houston, B.H.; Baldwin, J.W. Surface doping and band gap tunability in hydrogenated graphene. ACS Nano 2012, 6, 17–22. [Google Scholar] [CrossRef]
- Kumar, P.V.; Bernardi, M.; Grossman, J.C. The impact of functionalization on the stability, work function, and photoluminescence of reduced graphene oxide. ACS Nano 2013, 7, 1638–1645. [Google Scholar] [CrossRef]
- Mishra, M.; Joshi, R.K.; Ojha, S. Role of oxygen in the work function modification at various stages of chemically synthesized graphene. J. Phys. Chem. C 2013, 117, 19746–19750. [Google Scholar]
- Wong, C.H.; Ambrosi, A.; Pumera, M. Thermally reduced graphenes exhibiting a close relationship to amorphous carbon. Nanoscale 2012, 4, 4972–4977. [Google Scholar] [CrossRef]
- Chen, W.; Yan, L. Preparation of graphene by a low-temperature thermal reduction at atmosphere pressure. Nanoscale 2010, 2, 559–563. [Google Scholar]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Navarro, C.; Weitz, R.T.; Bittner, A.M.; Scolari, M.; Mews, A.; Burghard, M.; Kern, K. Electronic transport properties of individual chemically reduced graphene oxide sheets. Nano Lett. 2007, 7, 3499–3503. [Google Scholar] [CrossRef]
- Xiong, Z.D.; Chen, Y.; Zhong, S.X.; Wei, Y. Electrochemical reduction of graphene oxide films: Preparation, characterization and their electrochemical properties. Chin. Sci. Bull. 2012, 57, 3045–3050. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, X.; Zhang, J.; Boey, F.; Zhang, H. Direct electrochemical reduction of single-layer graphene oxide and subsequent functionalization with glucose oxidase. J. Phys. Chem. C 2009, 113, 14071–14075. [Google Scholar] [CrossRef]
- Cooper, D.R.; D’Anjou, B.; Ghattamaneni, N. Experimental review of graphene. ISRN Condens. Matter Phys. 2012, 2012, 501686:1–501686:56. [Google Scholar]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef]
- Gao, X.; Jang, J.; Nagase, S. Hydrazine and thermal reduction of graphene oxide: Reaction mechanisms, product structures, and reaction design. J. Phys. Chem. C 2009, 114, 832–842. [Google Scholar]
- Hwang, J.O.; Park, J.S.; Choi, D.S. Workfunction-tunable, n-doped reduced graphene transparent electrodes for high-performance polymer light-emitting diodes. ACS Nano 2011, 6, 159–167. [Google Scholar] [CrossRef]
- Chieh, Y.; Yan, W.; Chen, C.W. Work Function Evolution of Graphene Oxide by Utilizing Hydrothermal Treatment. In Proceedings of the 2010 8th International Vacuum Electron Sources Conference and Nanocarbon (IVESC), Nanjing, China, 14–16 October 2010; p. 552.
- Liu, X.; Kim, H.; Guo, L.J. Optimization of thermally reduced graphene oxide for an efficient hole transport layer in polymer solar cells. Org. Electron. 2013, 14, 591–598. [Google Scholar]
- Liu, J.; Xue, Y.; Dai, L. Sulfated graphene oxide as a hole-extraction layer in high-performance polymer solar cells. J. Phys. Chem. Lett. 2012, 3, 1928–1933. [Google Scholar] [CrossRef]
- Yang, D.; Zhou, L.; Chen, L.; Zhao, B.; Zhang, J.; Li, C. Chemically modified graphene oxides as a hole transport layer in organic solar cells. Chem. Commun. 2012, 48, 8078–8080. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; He, D.; Wu, H.; Wang, H.; Zhou, P.; Fu, M.; Jiang, K.; Chen, W. Organic photovoltaic devices based on an acceptor of solution-processable functionalized graphene. J. Nanosci. Nanotechnol. 2011, 11, 9432–9438. [Google Scholar] [CrossRef]
- Wang, H.; He, D.; Wang, Y.; Liu, Z.; Wu, H.; Wang, J. Organic photovoltaic devices based on graphene as an electron-acceptor material and P3OT as a donor material. Phys. Status Solidi 2011, 208, 2339–2343. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Z.; Zhang, X.; Zhang, N.; Yang, L.; Yin, S.; Chen, Y. Organic photovoltaic cells based on an acceptor of soluble graphene. Appl. Phys. Lett. 2008, 92, 223303:1–223303:3. [Google Scholar]
- Lin, Y.; Li, X.; Xie, D.; Feng, T.; Chen, Y.; Song, R.; Tian, H.; Ren, T.; Zhong, M.; Wang, K. Graphene/semiconductor heterojunction solar cells with modulated antireflection and graphene work function. Energy Environ. Sci. 2013, 6, 108–115. [Google Scholar] [CrossRef]
- Kang, B.; Lim, S.; Lee, W.H.; Jo, S.B.; Cho, K. Work-function-tuned reduced graphene oxide via direct surface functionalization as source/drain electrodes in bottom-contact organic transistors. Adv. Mater. 2013, 25, 5856–5862. [Google Scholar] [CrossRef]
- Kong, B.S.; Geng, J.; Jung, H.T. Layer-by-layer assembly of graphene and gold nanoparticles by vacuum filtration and spontaneous reduction of gold ions. Chem. Commun. 2009, 16, 2174–2176. [Google Scholar] [CrossRef]
- Goncalves, G.; Marques, P.A.A.P.; Granadeiro, C.M.; Nogueira, H.I.S.; Singh, M.K.; Grácio, J. Surface modification of graphene nanosheets with gold nanoparticles: The role of oxygen moieties at graphene surface on gold nucleation and growth. Chem. Mater. 2009, 21, 4796–4802. [Google Scholar]
- Li, J.; Yang, J.; Yang, Z.; Li, Y.; Yu, S.; Xu, Q.; Hu, X. Graphene-Au nanoparticles nanocomposite film for selective electrochemical determination of dopamine. Anal. Methods 2012, 4, 1725–1728. [Google Scholar]
- Zhang, Y.; Liu, S.; Wang, L.; Qin, X.; Tian, J.; Lu, W.; Chang, G.; Sun, X. One-pot green synthesis of Ag nanoparticles-graphene nanocomposites and their applications in SERS, H2O2, and glucose sensing. RSC Adv. 2012, 2, 538–545. [Google Scholar]
- He, H.K.; Gao, C. Graphene nanosheets decorated with Pd, Pt, Au, and Ag nanoparticles: Synthesis, characterization, and catalysis applications. Sci. Chin. Chem. 2011, 54, 397–404. [Google Scholar] [CrossRef]
- Wang, P.; Liu, Z.G.; Chen, X.; Meng, F.L.; Liu, J.H.; Huang, X.J. UV irradiation synthesis of an Au-graphene nanocomposite with enhanced electrochemical sensing properties. J. Mater. Chem. A 2013, 1, 9189–9195. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Ma, Z. Ionic liquid functionalized graphene/Au nanocomposites and its application for electrochemical immunosensor. Biosens. Bioelectron. 2013, 48, 33–38. [Google Scholar] [CrossRef]
- Roy, P.; Periasamy, A.P.; Liang, C.T.; Chang, H.T. Synthesis of graphene-ZnO-Au nanocomposites for efficient photocatalytic reduction of nitrobenzene. Environ. Sci. Technol. 2013, 47, 6688–6695. [Google Scholar]
- Lu, G.; Li, H.; Liusman, C.; Yin, Z.; Wu, S.; Zhang, H. Surface enhanced Raman scattering of Ag or Au nanoparticle-decorated reduced graphene oxide for detection of aromatic molecules. Chem. Sci. 2011, 2, 1817–1821. [Google Scholar] [CrossRef]
- Huang, X.; Li, S.; Wu, S.; Huang, Y.; Boey, F.; Gan, C.L.; Zhang, H. Graphene oxide-templated synthesis of ultrathin or tadpole-shaped Au nanowires with alternating hcp and fcc domains. Adv. Mater. 2012, 24, 979–983. [Google Scholar]
- Gu, H.; Yang, Y.; Tian, J.; Shi, G. Photochemical synthesis of noble metal (Ag, Pd, Au, Pt) on graphene/ZnO multihybrid nanoarchitectures as electrocatalysis for H2O2 reduction. ACS Appl. Mater. Interfaces 2013, 5, 6762–6768. [Google Scholar] [CrossRef]
- Hou, J.; Shao, Y.; Ellis, M.A.; Moore, R.; Yi, B. Graphene-based electrochemical energy conversion and storage: Fuel cells, supercapacitors and lithium ion batteries. Phys. Chem. Chem. Phys. 2011, 13, 15384–15402. [Google Scholar] [CrossRef]
- Subrahmanyam, K.S.; Manna, A.; Pati, S.K.; Rao, C.N.R. Graphene. Chem. Phys. Lett. 2010, 497, 70–75. [Google Scholar] [CrossRef]
- Lightcap, I.V.; Kamat, P.V. Graphitic design: Prospects of graphene-based nanocomposites for solar energy conversion, storage, and sensing. Acc. Chem. Res. 2012, 46, 2235–2243. [Google Scholar] [CrossRef]
- Kim, B.J.; Yang, G.; Kim, H.Y.; Hyeon, K.B.; Mastro, M.A.; Hite, J.K.; Eddy, C.R., Jr.; Ren, F.; Pearton, S.J.; Kim, J. GaN-based ultraviolet light-emitting diodes with AuCl3-doped graphene electrodes. Opt. Express 2013, 21, 29026–29030. [Google Scholar]
- Choe, M.; Cho, C.Y.; Shim, J.P.; Park, W.; Lim, S.K.; Hong, W.K.; Lee, B.H.; Lee, D.S.; Park, S.J.; Lee, T. Au nanoparticle-decorated graphene electrodes for GaN-based optoelectronic devices. Appl. Phys. Lett. 2012, 101, 031115:1–031115:5. [Google Scholar]
- Cho, C.-Y.; Choe, M.; Lee, S.J.; Hong, S.H.; Lee, T.; Lim, W.; Kim, S.T.; Park, S.J. Near-ultraviolet light-emitting diodes with transparent conducting layer of gold-doped multi-layer graphene. J. Appl. Phys. 2013, 113, 113102:1–113102:5. [Google Scholar]
- Kwon, K.C.; Kim, B.J.; Lee, J.-L.; Kim, S.Y. Effect of anions in Au complexes on doping and degradation of graphene. J. Mater. Chem. C 2013, 1, 2463–2469. [Google Scholar] [CrossRef]
- Sun, S.; Wu, P. Easy fabrication of macroporous gold films using graphene sheets as a template. ACS Appl. Mater. Interfaces 2013, 5, 3481–3486. [Google Scholar] [CrossRef]
- Koo, H.Y.; Lee, H.-J.; Noh, Y.Y.; Lee, E.S.; Kim, Y.H.; Choi, W.S. Gold nanoparticle-doped graphene nanosheets: Sub-nanosized gold clusters nucleate and grow at the nitrogen-induced defects on graphene surfaces. J. Mater. Chem. 2012, 22, 7130–7135. [Google Scholar]
- Jeon, E.K.; Seo, E.; Lee, E.; Lee, W.; Um, M.K.; Kim, B.S. Mussel-inspired green synthesis of silver nanoparticles on graphene oxide nanosheets for enhanced catalytic applications. Chem. Commun. 2013, 49, 3392–3394. [Google Scholar]
- Ran, X.; Sun, H.; Pu, F.; Ren, J.; Qu, X. Ag Nanoparticle-decorated graphene quantum dots for label-free, rapid and sensitive detection of Ag+ and biothiols. Chem. Commun. 2013, 49, 1079–1081. [Google Scholar]
- Dutta, S.; Ray, C.; Sarkar, S. Silver nanoparticles decorated reduced graphene oxide (rGO) nanosheets: A platform for SERS-based low level detection of uranyl ion. ACS Appl. Mater. Interfaces 2013, 5, 8724–8732. [Google Scholar] [CrossRef]
- Shim, J.P.; Kim, D.; Choe, M.; Lee, T.; Park, S.J.; Lee, D.S. A self-assembled Ag nanoparticle agglomeration process on graphene for enhanced light output in GaN-based LEDs. Nanotechnology 2012, 23, 255201:1–255201:6. [Google Scholar]
- Martínez-Orozco, R.D.; Rosu, H.C.; Lee, S.-W.; González, V. Understanding the adsorptive and photoactivity properties of Ag-graphene oxide nanocomposites. J. Hazard Mater. 2013, 263, 52–60. [Google Scholar] [CrossRef]
- Yang, G.; Li, Y.; Rana, R.K.; Zhu, J.-J. Pt-Au/nitrogen-doped graphene nanocomposites for enhanced electrochemical activities. J. Mater. Chem. A 2013, 1, 1754–1762. [Google Scholar] [CrossRef]
- Li, Y.; Gao, W.; Ci, L.; Wang, C.; Ajayan, P.M. Catalytic performance of Pt nanoparticles on reduced graphene oxide for methanol electro-oxidation. Carbon 2010, 48, 1124–1130. [Google Scholar] [CrossRef]
- Chen, X.; Su, B.; Wu, G.; Yang, C.J.; Zhuang, Z.; Wang, X.; Chen, X. Platinum nanoflowers supported on graphene oxide nanosheets: Their green synthesis, growth mechanism, and advanced electrocatalytic properties for methanol oxidation. J. Mater. Chem. 2012, 22, 11284–11289. [Google Scholar]
- Chen, C.; Long, M.; Wu, H.; Cai, W. One-step synthesis of Pt nanoparticles/reduced graphene oxide composite with enhanced electrochemical catalytic activity. Sci. Chin. Chem. 2013, 56, 354–361. [Google Scholar] [CrossRef]
- Tan, C.; Huang, X.; Zhang, H. Synthesis and applications of graphene-based noble metal nanostructures. Mater. Today 2013, 16, 29–36. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Garg, R.; Dutta, N.K.; Choudhury, N.R. Work Function Engineering of Graphene. Nanomaterials 2014, 4, 267-300. https://doi.org/10.3390/nano4020267
Garg R, Dutta NK, Choudhury NR. Work Function Engineering of Graphene. Nanomaterials. 2014; 4(2):267-300. https://doi.org/10.3390/nano4020267
Chicago/Turabian StyleGarg, Rajni, Naba K. Dutta, and Namita Roy Choudhury. 2014. "Work Function Engineering of Graphene" Nanomaterials 4, no. 2: 267-300. https://doi.org/10.3390/nano4020267
APA StyleGarg, R., Dutta, N. K., & Choudhury, N. R. (2014). Work Function Engineering of Graphene. Nanomaterials, 4(2), 267-300. https://doi.org/10.3390/nano4020267