Composites of Polymer Hydrogels and Nanoparticulate Systems for Biomedical and Pharmaceutical Applications
Abstract
:1. Hydrogels and Their Biomedical and Pharmaceutical Applications

2. Nanoparticles and Their Biomedical and Pharmaceutical Applications

3. Composites of Hydrogels and Nanoparticles
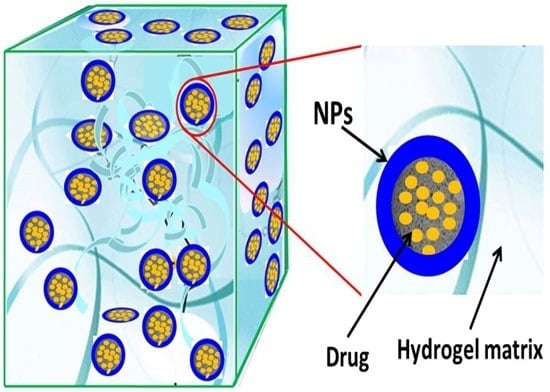
3.1. Nanocomposite Hydrogels: A Brief Overview


3.2. Nanocomposite Hydrogels from Hydrogels and Carbon-based NPs

| Fillers | Hydrogels | Applications and Functions | Ref. |
|---|---|---|---|
| Carbon Nanotubes (CNTs) | PVA | Drug delivery; Incorporation of CNTs caused a significant increase in tensile strength, decrease in elongation at break, and increase in Young’s modulus of the PVA hydrogels; Improved the electrical conductivity and antibacterial activities of the hydrogel systems; CNTs imparted to hydrogels the ability of electrical stimulation-controlled drug release | [105,106] |
| PHEMA | Tissue engineering; CNTs were added to improve electrical conductivity and mechanical properties; CNTs makes hydrogel more suitable as nerve conduits | [87] | |
| PNIPAM | Tissue engineering; Incorporation of CNTs could improve the bioactivities, especially supportive adhesion of PNIPAM to encapsulated cells and favor their efficacy in myocardial repair | [88] | |
| PAM | Biomaterials; CNTs can significantly improve the mechanical properties and swelling behavior of PAM hydrogels | [89] | |
| P(AM-co-EBA) | Drug delivery; CNTs was covalently included into films and acted as a functional element for both improving the electro-responsivity and modulating the release of anionic and cationic drugs | [90] | |
| P(EGDMA-co-HEMA) | Tissue engineering; CNTs can improve the swelling and mechanical properties | [107] | |
| P(VPB-co-DMA) | Biomaterials; CNTs could increase storage moduli of the hydrogel and endow the hydrogel with self-healing property | [96] | |
| MPEG/α-CD | Drug delivery; CNTs improved the hydrophobic drug loading amount; MPEG not only dispersed CNTs in aqueous solution, but also functioned as hydrogel matrix by interacting with α-CD | [108] | |
| GG-g-AA | Drug delivery; CNTs can increase the hydrophobicity of hydrogel, ensure the high environmental stability and drug retention properties of hydrogel | [109] | |
| BC/SA | Drug delivery; CNTs can be used to obtain the composite hydrogel with the highest electric sensitivity; Drug release from hybrid hydrogels showed a significant pulsatile characteristic | [110] | |
| DNA | Tissue engineering; Incorporation of CNTs significantly strengthens the mechanical properties of DNA hydrogel; DNA improved the biocompatibility of CNTs and the resulting hybrid hydrogel | [111] | |
| PEI-Fc | Drug delivery; CNTs can improve the electrical sensitivity properties of hydrogel and can be used to tune the stiffness and degree of swelling | [112] | |
| GEL | Tissue engineering; CNTs supported the contractile property of engineered cardiac tissues, improved the formation of gap junction and enhanced the excitation-contraction coupling | [113] | |
| CS | Antimicrobial biomaterials; The addition of CNTs in the composite hydrogel scaffold significantly reduced the water uptake ability, increased the antimicrobial activity and decreased the toxicity | [114] | |
| COL | Tissue engineering; CNTs enhanced the proliferative potential of the cells; Hydrogel offers excellent 3-D conditions for the growth of cells; CNTs within the hydrogel significantly stimulate cell secretion of neurotrophic factors and expression of neural markers | [115] | |
| Ambidextrous gelators | Biomaterials; The inclusion of SWNTs within the ambidextrous gels improved the mechanical rigidity of the resulting soft nanocomposites | [116] | |
| Peptide | Tissue engineering; Peptide enables the dispersion of the CNTs in aqueous media; CNTs make hybrid hydrogels have superior physical and mechanical properties | [117] | |
| Lysozyme | Drug delivery and tissue engineering; CNTs contributed to gelation, improved mechanical properties and improved the biological activity of hydrogels | [118] | |
| Graphene | PAA | Drug delivery; Graphene/PAA hydrogel was prepared with pH-sensitive, good thermal, strong mechanical and excellent elastic properties | [119] |
| PMAA | Drug delivery; The graphene hybrid hydrogels with enhanced mechanical, electrical, and thermal properties were prepared by in situ free radical polymerization, which exhibited controlled drug release in a pulsatile fashion upon the ON/OFF application of low electrical voltages | [120] | |
| PVA/P(MA-co-NIPAM) | Drug delivery; Small amounts of embedded graphene affect the behavior of mechanical and thermal properties of the hydrogel without altering its hydration state; The entrapment of graphene increase the possibility for functionalization and its dispersibility in water | [91] | |
| Bacterial cellulose | Tissue engineering; Graphene was used to reinforce various hydrogels, leading to excellent mechanical, electrical and biological properties; Graphene also changed the crystallinity of bacterial cellulose | [103] | |
| CS/GEL | Neural tissue engineering; Graphene improved the electrical conductivity and mechanical properties, but decreased the porosity, swelling ratio and in vitro biodegradability | [92] | |
| β-CD /PDMA | Drug delivery; β-CD endowed the self-healing property of hydrogel as anticancer drug carrier, more drug loading and better drug release behavior | [121] | |
| Graphene Oxide (GO) & reduced graphene oxide ( rGO) | PEG | Drug delivery; PEG endowed GO high aqueous solubility and stability in physiological solutions; GO imparted to hydrogel superior loading capacity for hydrophobic drug | [93] |
| PLA–PEG–PLA | Drug delivery; Hydrogel can serve as a sustained delivery depot of the drugs. GO can increase drug loading capacity | [94] | |
| PEGDGE | Drug delivery; Enhanced mechanical and electrical properties were observed with increased rGO content; Hydrogel can greatly improve the stability of rGO dispersions | [101] | |
| PAA/GEL | Tissue engineering; GO worked as reinforcement agent can significantly increase the tensile strength (71%) and elongation at break (26%) of composite hydrogels | [122] | |
| GEL | Drug delivery; GO worked as physical crosslinker, and the multiple crosslinking sites between gelatin chain and GO nanosheet rendered GO–gelatin hybrid hydrogels high mechanical performance; Drug release behaviors from these hydrogels could be manipulated by controlling the crosslinking density and pore sizes of the hydrogels | [123] | |
| α-CD/PEO-PPO | Drug delivery; The incorporation of GO enhanced the mechanical strength and stability of the hybrid hydrogels enormously; Hybrid hydrogels can really exhibit a sustained drug release effect | [102,124] | |
| Peptide | Drug delivery; Mechanical stability, syringe-injectability, low erosion rate, and near-infrared light triggered release functionalities | [99] | |
| KGM/SA | Drug delivery; GO as a drug-binding effector for anticancer drug loading and release; SA as the pH sensitive agent | [125] | |
| CS | Blood toxins adsorption; The incorporation of graphene oxide into the chitosan matrix improved both the Young’s modulus and the compressive strength of the hybrid hydrogel, as well as its adsorption capacity for bilirubin | [126] | |
| clay-PDMA | Biomaterial dressing; GO sheets in the hybrid hydrogels acted as not only a collaborative cross-linking agent but also as a NIR absorber to absorb the NIR irradiation energy and transform it to thermal energy rapidly and efficiently, resulting in a rapid temperature increase of the GO containing gels. | [127] | |
| Fullerene (C60) | PNIPAM | Drug delivery; The incorporation C60 improved the mechanical strength and decreased the swelling ratio and reduced the lower critical solution temperature of PNIPAM | [128] |
| PHEMA | Drug delivery; Equilibrium swelling ratio is lower due to the presence of hydrophobic fullerene moieties; The glass transition temperature and thermo-stability were increased with increasing fullerene content | [129] | |
| GC-g-DMA | Tumor therapy; The incorporation C60 endowed the hydrogel an advanced property for endosomal pH targeting and an elevated photodynamic tumor cell ablation at pH 5.0 through an enhanced singlet oxygen generation at pH 5.0 | [130] |
3.3. Nanocomposite Hydrogels from Hydrogels and Inorganic NPs or Semiconductor NPs



| Fillers | Hydrogels | Applications and Functions | Ref. |
|---|---|---|---|
| Silica (SiO2) particles or NPs | HA/PNIPAM | Tissue engineering; SiO2 NPs improve mechanical properties; HA can improve biocompatibility; The stability and the rigidity increase with the amount of PNIPAM | [159] |
| PNIPAM | Drug delivery and tissue engineering; SiO2 NPs improve the thermal and mechanical properties but decrease the swelling ratio | [160,161,162,163,164,165,166,167,168] | |
| PAM | Compression strength and elastic modulus of the composite hydrogels were significantly improved by adding SiO2 NPs compared with pure PAM hydrogels | [169,170,171,172,173] | |
| Silicone | Implantable medical devices; SiO2 can increase swelling behavior and improve stiffness of the hydrogel; Hybrid hydrogel can be used to decrease the protein absorption and the growth of bacteria | [174] | |
| PHEA | Drug delivery; The silica particles were well dispersed in hydrogels; The release rate of aspirin decreased with the increasing content of silica | [175] | |
| PVA | Biomaterials; The tensile strength of PVA hydrogel increased with SiO2 NPs content. Proper content of SiO2 NPs could also enhance the permeability and swelling property, resulting in the improvement of the capillary water absorption capacity of PVA hydrogel | [176] | |
| CS | Bone tissue engineering; SiO2 NPs have a positive effect on cell viability and induce the occurrence of mineralization not only at the surface of the material but also in its entire volume; CS as bioactive injectable systems for bone tissue repair can undergo in situ gelation under physiological temperatures | [177,178] | |
| Peptide | Biosensor; PEG/peptide can form enzyme responsive hydrogel and collapse in response to diverse biological stimuli; The negatively charged silica NPs used are also at a sufficiently high concentration to form a regular periodic structure within the hydrogel, acting as a photonic bandgap which can reflect visible wavelengths of light | [138,140] | |
| Cellulose | Tissue engineering and cell cultures; SiO2 preserves extracellular matrix-like materials and cellular proteins; The intact 3D spheroids can be recovered from the hydrogel by a cellulase enzyme for downstream applications | [179] | |
| PMAA or PAA | SiO2 as adhesive fillers that interacted with PMAA chains can improved viscoelastic moduli (up to 8.7 times) and enhanced elasticity | [180,181] | |
| ALG or COL | Tissue engineering; SiO2 can improve the mechanical properties, including surface roughness and hardness, of the hydrogel | [182,183,184] | |
| PEG | Drug delivery; Hydrogel can form sustained-release depot systems; Incorporation of SiO2 NPs can regulate the pores size, gelation time and viscosity of the hydrogel; The drug loading capacity and drug release profiles could be tuned | [185,186] | |
| MSNs | CS | Drug delivery; MSNs facilitate the drug loading and their subsequent release; CS enables pH-triggered drug release and improves the endocytosis of target cells and cell biocompatibility | [187] |
| Drug delivery; The introduction of MSNs into CS will facilitate the gelation process at body temperature and also promote the elastic modulus; MSNs can load small drug molecules and then steadily release them for a long time period | [178] | ||
| PClAETA | Biomaterials; Introduction of non-functionalized MSNs can improve the water absorption of the hydrogel | [188] | |
| Poly(aspartic acid) | Biomaterials; MSNs as crosslinker can optimize the pore morphology, improve thermal stability increase swelling ratio and enhance salt tolerance of the composite; MSNs can be uniformly and stably dispersed in the structure of the hydrogel | [189] | |
| PNIPAM | Biomaterials; MSNs can be used as effective “topological crosslinkers” to reinforce the PNIPAM hydrogels | [190] | |
| HAP and nBG | GEL | Bone tissue regeneration; HAP significantly improves the stiffness of GEL hydrogels, while it maintains their structural integrity and swelling ratio; Introduction of HAP results in a lower swelling ratio, higher mechanical moduli, and better biocompatibility and promotes cell functional expression for osteon biofabrication; Gelatin hydrogels also provide natural cell binding motifs, making them amenable for 3D cell encapsulation; GEL provide cell-responsive characteristics, cell adhesion sites, and proteolytic degradability | [191,192,193] |
| Silk fibroin | Bone tissue regeneration; HAP can increase compression modulus and mechanical properties, decrease the water uptake ability, improve metabolic and alkaline phosphatase activities of osteoblastic cells; Hydrogel plays regulatory role in oriented nucleation and growth of HAP crystals | [133,194,195,196,197] | |
| PAM | Tissue engineering; SiO2 can improve the mechanical properties, including surface roughness and hardness | [198] | |
| CS | Bone tissue regeneration; HAP enhances swelling, protein adsorption, exogenous biomineralization and osteoblast differentiation and also accelerates bone formation; Zn possessing excellent antimicrobial properties can tackle implant-associated microbial infections; CS can minimize immune response | [199,200,201,202] | |
| PVA | Bone tissue regeneration; Hydrogels are employed as a matrix gel in a mineralization solution; HAP was formed in the polymer matrix under mild conditions, mimicking mineralization in natural bone formation | [132,203,204] | |
| Pullulan | Bone tissue regeneration; Addition of HAP can improve compressive modulus of the scaffold, provide sites for cell adhesion, and render them osteoconductive in vitro; Hydrogels work as scaffolds for mineralization | [205] | |
| PECE | Tissue engineering; PECE endowed hydrogel good thermosensitivity and injectability; HAP can improve mechanical properties of hydrogel | [206] | |
| PEG | Bone substitute material; The formation of HAP in hydrogel matrices enable the acquisition of bioactive composites materials with desired shapes. | [207,208] | |
| ALG | Bone tissue engineering; Incorporation of nBG into hydrogel can combine excellent cellular adhesion, proliferation and differentiation properties, good biocompatibility and predictable degradation rates; ALG can increase lactate dehydrogenase and mitochondrial activity | [135,209,210,211] | |
| CHS | Bone tissue engineering; nBG shows an excellent stimulatory effect on bone formation; CHS improves integration of the nBG to prevent particle migration and promotes bone regeneration; The composite can encapsulate bone marrow to form a mechanically stable construct | [212] | |
| COL | Tissue engineering; Incorporation of nBG NPs improves mechanical stability and enhanced the proliferation rate and osteogenic differentiation | [136,213,214,215] | |
| LAP | GEL/PAM | Tissue engineering; Incorporation of LAP can enhance thermal stability, tensile and stretching properties; GEL can significantly improve the hydrogel’s pH-responsive properties and enhance the antithrombogenicity but decrease the degree of hemolysis of the gels | [216] |
| PEG/GEL | Tissue engineering; Hydrogel remain stable and provide a cell supportive microenvironment under normal cell culture conditions. LAP can preferentially induce osteogenic differentiation of human mesenchymal stem cells | [217] | |
| PEG | Tissue engineering; Incorporation of LAP significantly reduced the cure time while enhancing the adhesive and bulk mechanical properties of the hydrogel; PEG adsorbed onto LAP, forms a compact layer of mostly loops and trains on top of the nanoparticle and large loops around the edge of the particles; PEG elicits minimal inflammatory response and exhibits an enhanced level of cellular infiltration | [218,219] | |
| PAM | Tissue engineering; The incorporation of catechol on the PAM exhibits a strong affinity toward LAP and enhances stiffness and a viscous dissipation property | [220,221,222] | |
| P(MEOMA-OEGMA) | Drug delivery and tissue engineering; LAP as physical cross-linker had significant influence on the microstructure and swelling/deswelling behaviors of hydrogels.; OEGMA can increase equilibrium swelling ratio and water retention; Hydrogel can provide thermosensitivity and the excellent biocompatibility | [223] | |
| P(AM-DMAEMA) | Tissue engineering, cell culture substrates and biosensors; LAP serves as a physical cross-linker and can change the mechanical strength of the hydrogel under direct-current electric field | [224] | |
| PNIPAM | Tissue engineering; LAP can influence the polymeric chain arrangement and increase the mechanical toughness and thermal stability | [225,226] | |
| Pluronic F127 | Tissue engineering; The interactions between LAP and Pluronic F127 may contribute to rearrangements of network structures at high deformations, leading to high elongations and improved toughness | [227] | |
| GMA | Biomaterials; LAP can reinforce mechanical toughness and elasticity; GMA regulates equilibrium swelling ratio and improves water content; Integrating LAP and GMA can improve self-standing ability and rheological, compression, and tensile properties | [228] | |
| MMT | CS | Drug delivery; MMT can enhance the loading of positively charged drug and affect the hydrogel’s drug release mechanism and swelling properties; The hydrogel can be used for controlled- release of drugs | [137,229] |
| CS-g-PAM | Superabsorbent polymer composites; Incorporation of MMT can optimize their absorption capacity, improve their swelling rate and salt-resistant ability; Composites exhibit moderate antibacterial activity in acidic medium | [230] | |
| PAM | Drug delivery; MMT effects the equilibrium swelling and drug release behavior of the composite; MMT can improve the barrier property of nanocomposite hydrogels and decrease the burse effect. MA effects the pH-responsivity on equilibrium swelling and release of drug | [231,232,233] | |
| P(ATC-AM) | Drug delivery; MMT serves as chemical cross-linker to enhance strength and toughness and decrease the swelling degree; ATC is cationic monomer and exist cation- exchange reaction with MMT | [234] | |
| GEL or COL | Drug delivery and wound dressing; Drug intercalation results in changes in MMT layered space; Integration of drug loaded MMT and gelatin creates biodegradable composite hydrogels with controlled drug release property, improved mechanical and thermal properties | [235,236] | |
| PAA or PMA | Biomaterial implants; MMT is used for adsorbing drug, achieving high drug loading; PAA is used for pH/bacteria- responsive releasing | [237] | |
| Polysaccharide | MMT can enhance strength and toughness and decrease the swelling degree | [238] | |
| PNIPAM | Tissue engineering; MMT can influence the polymeric chain arrangement and the pore size and increase the complex viscosity and adhesion strength as well as thermal stability | [239,240,241,242,243] | |
| Other silicate particles | P(AM-IA) | Drug delivery; Incorporation of hectorite offers the hydrogel with suitable water absorbency, shear-resistance, high gel strength and good thermal stability; IA provides pH-responsivity on drug release | [244,245,246] |
| ALG | Drug delivery; Halloysite nanotubes improve complex surface topography and structural integrity and achieve a sustained release of the growth factor; The composites enhance repair and regeneration in damaged or diseased tissues | [139] | |
| PEG/P(AA-VP) | Drug delivery; Phyllosilicate enhances the water uptake with a desirable strength; Hydrogels are used for pH responsible and controlled release | [247] | |
| PCL-PEG-PCL | Bone regeneration; Mesoporous magnesium silicate can enhance the compressive strength, elastic modulus, and hydrophilicity of hydrogel, and promote cell attachment and proliferation and increase the degradability of the hydrogel | [248] | |
| PAM | Tissue engineering; Attapulgite nanorods grafted with vinyl groups serve as macro-crosslinkers, which can significantly increase the modulus, strength, and toughness of hydrogels; | [249] | |
| P(AA-NIPAM) | Tissue engineering; The presence of imogolite nanotubes produced strong hydrogels that exhibit thermo-responsive volume transition because of the coil/globule transition of PNIPAM chains. | [250] |
3.4. Nanocomposite Hydrogels from Hydrogels and Metal NPs or Metal Oxide NPs


| Fillers | Hydrogels | Applications and Functions | Ref. |
|---|---|---|---|
| Ag NPs | PAM | Wound dressing; Ag NPs demonstrate excellent antibacterial activity; Polymer hydrogels were used to keep the NPs stable and contact with skin | [254,255,293,294,295,296,297] |
| Biosensor; Ag NPs can change the strength of the localized surface plasm on resonance by regulating interparticle distances; PAM can be swelling with ionic strength change | [256] | ||
| PVP or PVA | Wound dressing or tissue engineering; Ag NPs are antibacterial agents and enhance the mechanical and thermal strength of the hydrogel; Hydrogels work as an efficient stabilizer of Ag NPs and control the release of the Ag NPs | [257,258,298,299,300,301,302] | |
| PNIPAM | Antibiotic materials; Ag NPs work as antimicrobial agents; PNIPAM is temperature-responsive component and make Ag NPs stable and uniformly distributed | [259,303,304,305,306] | |
| PEGDMA | Contact lenses; Ag NPs work as antimicrobial agents | [307] | |
| Acrylate-based copolymers | Biosensors and drug delivery; Ag NPs work as antimicrobial agents; Copolymer hydrogel with different swelling properties is used to disperse Ag NPs | [262,266,308,309,310] | |
| Ophthalmic lenses; The addition of Ag NPs is associated with the reduction of UV-B transmittance and increase in tensile strength | [311] | ||
| AMPS-Na | Burn wound dressing; Silver hydrogel is efficient at preventing bacterial colonization of wounds and has good inhibitory action against bacteria | [263,312] | |
| NaDOC | Antibacterial materials; Ag NPs are antibacterial agents; NaDOC can immobilize Ag NPs via pH-induced self-assembly | [313] | |
| Graphene | Wound dressing; Ag NPs are antibacterial agents; Graphene is conductive to cellular adhesion and growth; The composite can significantly accelerate the healing rate of artificial wounds | [264] | |
| Silicone | Adhesion to catheters to prevent urinary tract infections; Ag NPs work as antimicrobial agents | [265] | |
| Lysine gelator | Nanomedicine; Gelator exhibits good gelation ability, low haemolytic activity and high biocompatibility to mammalian cells; Incorporation of Ag NPs exhibits excellent antibacterial activity and significant mechanical strength | [314] | |
| Peptides | Wound healing and sustained Ag NPs release; Ag NPs inhibit bacterial growth; Peptide fibers prevent aggregation of Ag NPs and improve the biocompatibility of the system | [267] | |
| Antibacterial materials and bioimaging; Fluorescent Ag NPs show excellent optical properties and antibacterial activity; Peptides make the Ag NPs stable | [315] | ||
| PAA | Drug delivery; PAA is used to control the release of antibacterial drugs; Ag NPs can enhance the antibacterial activity | [316] | |
| Cellulose | Wound dressing and antimicrobial materials; Ag NPs show high antibacterial activity against Gram positive and Gram negative bacteria; Cellulose serves as a gelling agent as well as a reducing agent for AgNO3 and a stabilizer for Ag NPs | [247,261,317] | |
| GEL or COL | Wound dressing; Ag NPs are antibacterial agents; Hydrogel is used for stabling Ag NPs | [268,318,319] | |
| GEL/CS | Drug delivery and tissue engineering; Benzotriazole maleimide functionalized Ag NPs work as cross-linkers, improve the storage modulus and decrease swelling ratios | [320] | |
| AG-CS | Biosensor; Ag NPs improve the conductivity and mechanical strength of the hydrogels and also have antimicrobial activity; Biopolymer moieties act as both reducing and stabilizing agents | [321] | |
| AG | Antibiotic materials; Hydrogels promote the Ag NPs protection and inhibit their aggregation; Ag NPs can produce Ag + to kill bacteria | [322,323] | |
| Au NPs | PNIPAM | Drug delivery; Au NPs can be used as a photoabsorbing agent to generate heat from optical energy; PNIPAM is thermo-responsive to control the release of drugs | [270,324,325,326] |
| Biosensor for the detection of DNA; Au NPs immobilize DNA strands | [278] | ||
| PEG | Tissue engineering; RGD modified gold nanoarrays as scaffold to control cell localization and differentiation | [279] | |
| Drug delivery; Au NPs are utilized as building blocks; α-CD and PEG form temperature-responsive reversible supramolecular hydrogel through host–guest interaction | [271] | ||
| PEGDA | Tumor therapy; Au NRs can be used as a photoabsorbing agent to generate heat from optical energy; The introduction of Au NRs is conducive to the formation of the hydrogel and accelerates the rate of cytotoxic singlet oxygen generation; PEGDA can stabilize Au NRs | [275] | |
| PPy | Clinical diagnosis; Au NPs can further increase the specific surface area to capture a large amount of antibodies as well as improve the capability of electron transfer | [327] | |
| PEDOT | Electroporation-assisted cell uptakes; Au NPs work as microelectrode; PEDOT modification is effective in preventing electrolysis during the electroporation | [328] | |
| CS | Drug delivery; Au NPs act as a role of physical cross-linking agents; This physical cross-linked hydrogel shows excellent drug loading ability | [272] | |
| GEL | Bone tissue engineering; The hydrogels loaded with Au NPs promote proliferation, differentiation, and alkaline phosphate activities of human adipose-derived stem cells and have a significant influence on new bone formation; GEL is a photo-curable hydrogel to embed Au NPs | [280] | |
| Peptide | Tissue engineering; Au NPs act as cross-linking agents and strengthen the prepared hydrogel | [329] | |
| DNA | Drug delivery and photothermal therapy; Au NPs can generate heat upon laser excitation and be used for photothermal therapy; DNA hydrogel can be fragmented by heat generations to release drugs and Au NPs | [273] | |
| DNAzyme | Detection of lead; Au NPs are used for visual detection; DNAzyme can be activated by lead and conducive to dissolve the hydrogel to release Au NPs | [330] | |
| Graphene | Biosensor for nitric oxide detection; Au NPs catalyze the electrochemical oxidation of NO; Graphene is used for uniformly depositing Au NPs | [331] | |
| Fe3O4 NPs | κ-carrageenan | Drug delivery; Fe3O4 NPs offer the hydrogel with magnetic-responsiveness properties; κ-carrageenan is pH responsive | [332,333,334,335] |
| Starch | Drug delivery; Fe3O4 NPs endow the hydrogel with magnetic sensitivity; The composite also shows temperature/pH sensitivity | [336,337,338] | |
| Cellulose | Drug delivery; Magnetic nanoparticles are used as cross-linkers; The release of DOX is significantly enhanced at external magnetic field | [339,340] | |
| Fibrin | Tissue engineering; Fe3O4 NPs make the hydrogel show excellent hyperthermia properties under exposure to an alternating magnetic field; The presence of Fe3O4 NPs in the fibrin can improve cell adhesion and colonization of osteogenic cells | [282,341] | |
| HA | Magnetic resonance imaging and drug delivery; HA can be degraded by hyaluronidase and release hydrophobic drugs; Fe3O4 NPs serve as imaging agents to track the hydrogel degradation and degradation products in vivo | [342] | |
| CS | Drug delivery; The presence of Fe3O4 NPs can make the hydrogels have magnetic response property and deliver and release encapsulated anticancer agent at the tumor by the weak magnetic field | [343,344,345] | |
| ALG | Cell encapsulation; Alginate cell capsules with Fe3O4 NPs can be easily controlled and manipulated by external magnetic fields | [346] | |
| COL-HA-PEG | Cartilage tissue engineering; The presence of Fe3O4 NPs can make the hydrogel travel to the tissue defect sites under remote magnetic guidance | [288] | |
| Guar gum | Drug delivery and theranostic; The NPs work as imaging agents and produce hyperthermia; Aminated guar gum can form the hydrogel without using toxic crosslinking agents | [347] | |
| PPZ | Drug delivery and biodetection; Ferrite nanoparticles is used for long-term magnetic response theragnosis; The composite can be used for sustainedly releasing cargos | [348] | |
| PAM | Drug delivery and protein separation; The presence of Fe3O4 NPs can make the hydrogel exhibit higher dielectric constants and magnetic responsiveness; The composite also has the properties of fast adsorption | [349,350,351] | |
| Drug delivery; Incorporation of Fe3O4 NPs endows the hydrogel with the ability of direction-dependent thermogenesis in an alternating magnetic field; The novel magnetic hydrogel shows a direction-dependent release of drugs that has a 3.4-fold difference between the two directions | [352] | ||
| PNIPAM | Drug delivery; Incorporation of magnetic nanoparticles endows the hydrogel with on-demand pulsatile drug release triggered by alternating magnetic field | [353] | |
| PVP or PVA | Drug delivery; Fe3O4 NPs are used for magnetic drug targeting; The hydrogel is used for carry cancer therapeutic agent | [354,355,356] | |
| PEGDA | Cell patterning; Incorporation of Fe3O4 NPs can manipulate space pattern of the hydrogel; PEGDA blocks are used as a stencil to define the area for cell adhesion and the second types of cells could be seeded after the magnetic block was removed to create heterotypic cell patterns | [357] | |
| Acrylate/acrylamide-based copolymers | Drug delivery and protein immobilization; Fe3O4 NPs endow the hydrogel with properties of magnetic stimuli-responsive; The copolymer hydrogel exhibits pH- and thermosensitive properties | [88,358,359,360,361] | |
| P(HEMA-GMA)-PANI | Immobilization of glucoamylase; Fe3O4 NPs show super-paramagnetism and make the hydrogel easily dispersed or separated from the medium without needing high magnetic intensity; Grafting of polyaniline on the hydrogel increases 3.4 times maximum adsorption capacity | [362] | |
| Cu or CuO NPs | PVP/PVA | Bioimaging and drug delivery; Cu NPs with red emission properties are used for optical imaging as well as for flow cytometric probe of cellular uptake; Cu NPs also generate reactive oxygen species to enhance the efficiency of killing cancer cells; PVP is used for stabling the NPs and delivering drugs | [363] |
| PEGDA | Antibacterial material; Cu NPs-modified hydrogel can be used for preventing bacterial infections | [364] | |
| Cellulose | Antibacterial material; CuO NPs endow the hydrogel with antibacterial activity | [365] | |
| ZnO NPs | PAM | Wound dressing; ZnO NPs endow the hydrogel with antibacterial activity; PAM provides uniform distribution and binding of the NPs to the fiber surface and to prevent their agglomeration | [366] |
| Cellulose | Antibacterial materials; ZnO NPs endow the hydrogel with antibacterial activity; The composite shows a pH and salt sensitive swelling behavior | [367,368] | |
| Chitin | Wound dressing; ZnO NPs endow the hydrogel with antibacterial activity and blood clotting ability; Incorporation of ZnO NPs improve healing rate and collagen deposition ability of composite bandages | [369] | |
| ZnS NPs | PHEMA/PAA | Artificial cornea implants; ZnS NPs can improve the refractive index of the polymer | [370] |
| Pt NPs | Polyimide | Biosensor; Pt NPs work as microelectrades; The composite works as microsensor to detect glutamate in vivo | [371] |
| Ni NPs | Cellulose | Responsive biomaterials; Ni NPs offer the gel with magnetic response properties with can be changed by temperature and the size of the NPs | [289] |
| Co NPs | PHEMA | Tissue engineering; Co NPs enable the hydrogel that combine the use of saturation magnetizations and high particle loading, flexibility, and shape memory; PHEMA can reduce magnetic particle migration or loss | [290] |
| MnO2 NPs | CS | Biosensor; MnO2 NPs work as catalytic for H2O2; The composite can be used for detections of choline chloride | [372] |
| TiO2 NPs | PAM/PNIPAM | Responsive biomaterials; Modified TiO2 NPs serve as cross-linker and enhance the mechanical strength; The hydrogel bilayer is able to bend to opposite directions to form hydrogel circles in pure water and 20% NaCl solution | [373] |
3.5. Nanocomposite Hydrogels from Hydrogels and Polymeric NPs or Liposomes
| Fillers | Hydrogels | Applications and Functions | Ref. |
|---|---|---|---|
| PLGA particles or NPs | PVA | Loading and delivery of biomacromolecular drugs; PLGA can increase drug loading and decrease burst release; Hydrogel can prevent the foreign body reaction(FBR), promote angiogenesis around subcutaneous implants and extend the lifetime of implantable biosensors | [375,376] |
| Drug delivery; PVA hydrogel acts as a hydrophilic base to support the microspheres; PLGA microspheres serve as drug reservoirs to continuously drug release | [377,378,379,380,381] | ||
| ALG | Drug delivery; Maintain consistent release of rhBMP-2; Improve bone formation and osseous integration | [382] | |
| PuraMatrixTM peptide | Drug delivery; PuraMatrixTM peptide hydrogel as vaccine adjuvants to recruit and activate immune cells | [383] | |
| F127 | Drug delivery; Sustained release of protein drugs; Temper burst release and prolong delivery of drugs at the site of a spinal cord injury | [384,385] | |
| P407 | Tissue engineering; Preserve protein structure and integrity, allowing a better and prolonged release profile and the maintenance of their biological activity. Hydrogel further protected it from the hydrophobic environment | [386] | |
| ALG | Cell-based tissue engineering; Offer a continuous and localized release of drug; Provide a physical support for microcapsules, facilitating administration, ensuring retention and recuperation and preventing dissemination; Reduce post-transplantation inflammation and foreign body reaction, thus prolonging the lifetime of the implant | [387] | |
| PEI-PEGDA | Drug delivery; Hydrogels are used for carrying dual or multi-molecular compounds and releasing them in a bimodal, sequential manner | [388] | |
| HAMC | Drug delivery; HAMC hydrogel is well-tolerated, having a minimal inflammatory response; NPs offer sustained release while the HAMC gel localizes the NPs at the site of injection | [389,390,391,392,393,394,395] | |
| PAM | Antivirulence treatment of local bacterial infection; RBC-coated NPs is an effective detoxification platform against bacterial infections; Hydrogels preserve the structural integrity and the functionalities of the contained NPs and offer additional engineering flexibility to improve the therapeutic efficacy | [396] | |
| MC | Drug delivery; Particle localization, decreases initial burst, and further prolongs release | [397] | |
| GG | Sustained local delivery of drug; For the local treatment of osteoporosis and other bone tissue disorder; Improve the low bioavailability and decrease the high toxicity of sodium alendronate | [398] | |
| PNIPAM | Drug delivery; PLGA used for the isolation of the drug, a slower drug-release rate, and the achievement of different drug release profiles | [399] | |
| PAMAM | Drug delivery; The residence time of pilocarpine can be prolonged by hydrogel; PLGA nanoparticles are safe for delivery of ophthalmic agents and are capable of sustained delivery of antiglaucoma agents | [400] | |
| Fmoc-peptide | Drug delivery and tissue engineering; Peptide hydrogel will likely be more stable in vivo; Sustained release of drug | [401] | |
| DBM-BHPEA NPs | Silicone | Protect the eyes from UV rays; Increase the pore size, allowing the particles to diffuse into the lenses. | [402] |
| HPMC-PEG NPs | Branched PAA | Drug delivery; Optimize drug therapies in which the release of hydrophobic compounds; Selectively direct to specific cell lines; Able to stay localized at the injection site | [403] |
| Silk fibroin particles | Silk fibroin | Tissue engineering; Offering well suited rheological features for injectability, and shape conformability into defect sites as well as controlled delivery rate | [404] |
| PHBV NPs | GG | Drug delivery; Minimally invasive administration and the controlled delivery of the active agent | [405] |
| PEG-PLA NPs | HPMC | Drug delivery; Shear-thinning and self-healing properties; Dual loading of a hydrophobic molecule into the NPs and a second hydrophilic molecule into the aqueous bulk of the gel, and thus enabled simultaneous release of both hydrophobic and hydrophilic drug in vivo from a single gel. | [406] |
| PPy NPs | ALG | Drug delivery and tissue engineering; PPy is stable in solution over the period of a month, and have good drug loading capacity; Localized depot releasing systems; Pulsatile releasing behaviors and maintain morphology and mechanical strength. | [407,408] |
| PHBHHx NPs | CS/GP | Drug delivery; Relatively strict control of long-term insulin release | [409] |
| PGT NPs | Silicone | Drug delivery in eye; Increase patient compliance and the bioavailability; Increase the release duration from the contact lenses; Establish safety and efficacy of glaucoma therapy by extended wear of nanoparticle loaded contact lenses | [410] |
| CS NPs | GX | Scaffold system for dual growth factor delivery in bone regeneration; Develop an in situ gelling biomaterial combining the viscoelasticity of natural polymers with the powerful antimicrobial properties of chitosan | [411] |
| PMMA NPs | Polysaccharide-PAA | Drug delivery; Hydrogels can remain localized at the site of injection, showing high biocompatibility and good ability to provide short term delivery. PMMA based NPs can be traced both in vitro and in vivo biological studies over a long period of time without side effects due to the biodegradation process | [412] |
| PβAE NPs | PAMAM-DEX | siRNA delivery; High transfection efficiency and low cytotoxicity; Sustained delivery of the siRNA; Enhance the stability of the NPs | [413] |
| PLA particles | PECE | Drug delivery and tissue engineering; Increase the thickness of the corium; Increase cell adhesion of microspheres | [414] |
| PAMAM dendrimer | CS-PEG | Drug delivery; Dendrimer was used to increase the solubility, loading efficiency and homogeneity of hydrophobic drug; Hydrogel provide a local drug delivery depot for a prolonged drug release | [415] |
| COL | Drug delivery; Dendrimer can improve the biostability and structural integrity of COL, which can make COL have higher denature temperature and resistance against collagenase digestion | [416] | |
| HA | Biofabrication; Form a fast cross-linking hydrogel; Improved the cell viability, proliferation, and attachment | [417] | |
| PEG-LA-DA | Tissue engineering; The multiple cross-linking sites present on the dendrimers can increase the cross-linking density at lower concentrations; The spherical dendrimers may provide discrete “molecular islands” in the network to limit swelling and improve mechanical properties; The multiple end-groups on the dendrimers facilitate the introduction of functional groups into the system at the nanoscale level | [418] | |
| Carbopol 980 | Drug delivery; PAMAM dendrimers strongly affects their influence on the improvement of solubility and antifungal activity of drug | [419] | |
| PEG | Tissue engineering and drug delivery; Hydrogel crosslinked with dendrimers, showing improved cytocompatibility, controlled swelling and degradation | [420] | |
| Solid lipid NPs | Poloxamer | Drug delivery; NPs can increase gel strength and mucoadhesive force; Easy to administer rectally; Gelled rapidly inside the body; Increased dissolution rate of the drug; Reduced initial burst effect | [421] |
| Liposomes | PAM | Drug delivery and antibacterial; Hydrogel can stabilize liposomes against fusion and preserves the structural integrity of the liposomes; The hydrogel formulation allows for controllable viscoeleasticity and tunable liposome release rate | [422] |
| Peptide | Tissue regeneration; Liposomes can enhance binding of growth factors to peptide fibers of the gel matrix and achieve delayed release; Bimodal drug release | [423] | |
| HA | Drug delivery; Strengthen the network formed by HA chains, high efficiency encapsulation, easily injectable and less invasive, delay and control the release of drugs for local delivery | [424,425,426,427] | |
| PNIPAM | Drug delivery; The intact liposomes with their content can be controlled to release from hydrogel by changing the temperature, which can be used for temperature-triggered on-demand delivery | [428] | |
| PVA | Tissue engineering; Hydrogel can retain the structural integrity and contents of liposomes | [429] | |
| PVP-PAA-PBMA | Drug delivery, Hydrogel can ensure liposomes original vesicle structure; Liposomes can improve viscoelastic features and drug release profiles | [430] | |
| Micelles | PECA | Drug delivery; Improve docetaxel solubility and permeability; The pH-responsive hydrogel controlled the micelles diffusion excellently in intestinal environment, thus achieving the target delivery of drug-loaded micelles to small intestine and significantly improvement of the oral bioavailability of docetaxel | [431] |
| PEG-PCL-PEG | Drug delivery and wound dressing; High drug loading and encapsulation efficiency; Improve combined curcumin solubility and permeability; Exhibit excellent wound healing activity and promote tissue reconstruction processes | [432] | |
| Agarose | Drug delivery; Achieve functional hydrogels capable of stimulus-triggered drug release | [433] | |
| PEG | Gene delivery; The incorporation of the nanosized micelles provided an excellent mean to tune physical properties of the hydrogels, such as increasing porosity and tunable mechanical property of the hydrogels and providing the best balance among hydrogel stiffness and porosity for cell survival | [434] | |
| Nanogels or microgels | PEG | Tissue engineering; Hydrogel can control the degradation and release of nanogels; Possess relatively strong mechanical properties, biodegradability; Nanogels can trap proteins by hydrophobic interactions; Sustainedly release proteins in their native form | [435] |
| PAM | Obtain macroscopic hydrogels with a fast response to external stimuli; Obtain strong hydrogel matrixes and composite gels with an internal structure | [436] | |
| PEG | Drug delivery; Multidrug delivery system; High drug loading and encapsulation efficiency; Encapsulate various hydrophobic substances | [437] | |
| DEX | Drug delivery; Both proteins and poorly water-soluble low-molecular-weight drugs can be efficiently encapsulated in the nanogels; Eliminating the initial burst release; Offer a maximum pharmacological efficiency at a minimum drug dose, reducing administration frequency and improving patient compliance | [438] | |
| Carbohydrate | Drug delivery; Improve loading or prolong delivery of a target drug; Prevent migration of the microgels from target sites; Provide an additional diffusive barrier for drug release; Reducing burst release effects and prolonging drug release kinetics | [439] | |
| CHP nanogel | Hyaluronan | Drug delivery; Minimize denaturation by trapping the protein in a hydrated polymer network; Simultaneously achieved both simple drug loading and controlled release with no denaturation of the protein drugs | [440] |
| Vesicles | CS | Functional biomaterials; The vesicle serves as reversible “dynamic” crosslinks that hydrophobic chains can be either embedded into the bilayers of vesicle or pulled out; Vesicles can afford multi-action sites for hydrophobic interaction, indicating that the self-healing rate of such a hydrogel would be much faster | [441] |
| PVA | Drug delivery; The vesicles can be evenly dispersed in PVA and are stable. The release can be triggered and the calcein diffuses afterwards quickly throughout the gel | [442] | |
| Xanthan | Drug delivery; The hydrogel shows a protective effect on vesicle integrity and leads to a slow release of the loaded model molecules from the polysaccharidic system; The vesicular structures may enhance the delivery of drug in the stratum corneum due to their specific constituents | [443] | |
| Micro-droplet | ALG | Drug delivery and bone tissue engineering; Facilitate the regional regulation of encapsulated cell fate; In situ formation of localized, sustained bioactive molecule delivery to encapsulate stem cells for therapeutic applications | [444] |
| Virus | ALG | Regenerative medicine; Significant improvement in cell attachment; Mimic the biological niche of a functional tissue; Localization, delivery, and differentiation of stem cells | [445] |




4. Conclusions and Future Perspective
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Li, Y.; Rodrigues, J.; Tomas, H. Injectable and biodegradable hydrogels: Gelation, biodegradation and biomedical applications. Chem. Soc. Rev. 2012, 41, 2193–2221. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th Anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv. Mater. 2014, 26, 85–124. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Seliktar, D. Designing cell-compatible hydrogels for biomedical applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Uragami, T.; Nakamae, K. Biomolecule-sensitive hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 79–98. [Google Scholar] [CrossRef]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 49–60. [Google Scholar] [CrossRef]
- Hoffman, A.S. Stimuli-responsive polymers: Biomedical applications and challenges for clinical translation. Adv. Drug Deliv. Rev. 2013, 65, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive polymers for biomedical applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Wichterle, O.; Lim, D. Hydrophilic gels for biological use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Zhu, J.M. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C. Recent advances in crosslinking chemistry of biomimetic poly(ethylene glycol) hydrogels. RSC Adv. 2015, 5, 39844–39853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maitra, J.; Shukla, V.K. Cross-linking in Hydrogels—A Review. Am. J. Polym. Sci. 2014, 4, 25–31. [Google Scholar]
- Ebara, M.; Kotsuchibashi, Y.; Narain, R.; Idota, N.; Kim, Y.-J.; Hoffman, J.M.; Uto, K.; Aoyagi, T. Smart Hydrogels. In Smart Biomaterials; Ebara, M., Kotsuchibashi, Y., Narain, R., Idota, N., Kim, Y.J., Hoffman, J.M., Uto, K., Aoyagi, T., Eds.; Springer: Tokyo, Japan, 2014; pp. 9–65. [Google Scholar]
- Huang, P.; Wang, W.; Zhou, J.; Zhao, F.; Zhang, Y.; Liu, J.; Liu, J.; Dong, A.; Kong, D.; Zhang, J. Amphiphilic polyelectrolyte/prodrug nanoparticles constructed by synergetic electrostatic and hydrophobic interactions with cooperative pH-sensitivity for controlled doxorubicin delivery. ACS Appl. Mater. Interfaces 2015, 7, 6340–6350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lin, X.; Liu, J.; Zhao, J.; Dong, H.; Deng, L.; Liu, J.; Dong, A. Sequential thermo-induced self-gelation and acid-triggered self-release process of drug-conjugated nanoparticles: A strategy for sustained and controlled drug delivery to tumor. J. Mater. Chem. B 2013, 1, 4667–4677. [Google Scholar] [CrossRef]
- Thomas, V.; Namdeo, M.; Mohan, Y.M.; Bajpai, S.K.; Bajpai, M. Review on polymer, hydrogel and microgel metal nanocomposites: A facile nanotechnological approach. J. Macromol. Sci. Pure Appl. Chem. 2008, 45, 107–119. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, G. Novel nanocomposite hydrogels consisting of layered double hydroxide with ultrahigh tensibility and hierarchical porous structure at low inorganic content. Adv. Mater. 2014, 26, 5950–5956. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Martin, C.; Kostarelos, K.; Prato, M.; Vazquez, E. Nanocomposite Hydrogels: 3D Polymer-Nanoparticle Synergies for On-Demand Drug Delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, L.M.; Segal, E. Nanostructured porous silicon-polymer-based hybrids: From biosensing to drug delivery. Nanomedicine 2011, 6, 1755–1770. [Google Scholar] [CrossRef] [PubMed]
- Ye, E.; Loh, X.J. Polymeric Hydrogels and Nanoparticles: A Merging and Emerging Field. Aust. J. Chem. 2013, 66, 997–1007. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Peppas, N.A.; Khademhosseini, A. Nanocomposite hydrogels for biomedical applications. Biotechnol. Bioeng. 2014, 111, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Deng, L.; Zhang, J.; Yin, L.; Dong, A. Composites of electrospun-fibers and hydrogels: A potential solution to current challenges in biological and biomedical field. J. Biomed. Mater. Res. B 2015. [Google Scholar] [CrossRef] [PubMed]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle-hydrogel composites: Concept, design,and applications of these promising, multi-functional materials. Adv. Sci. 2015, 1–2. [Google Scholar] [CrossRef]
- De, M.; Ghosh, P.S.; Rotello, V.M. Applications of Nanoparticles in Biology. Adv. Mater. 2008, 20, 4225–4241. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Green, L.A.W. Functionalisation of nanoparticles for biomedical applications. Nano Today 2010, 5, 213–230. [Google Scholar] [CrossRef]
- Kim, T.; Hyeon, T. Applications of inorganic nanoparticles as therapeutic agents. Nanotechnology. 2014, 25. [Google Scholar] [CrossRef] [PubMed]
- Doane, T.L.; Burda, C. The unique role of nanoparticles in nanomedicine: Imaging, drug delivery and therapy. Chem. Soc. Rev. 2012, 41, 2885–2911. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 2013, 42, 1147–1235. [Google Scholar] [CrossRef] [PubMed]
- Arvizo, R.R.; Bhattacharyya, S.; Kudgus, R.A.; Giri, K.; Bhattacharya, R.; Mukherjee, P. Intrinsic therapeutic applications of noble metal nanoparticles: Past, present and future. Chem. Soc. Rev. 2012, 41, 2943–2970. [Google Scholar] [CrossRef] [PubMed]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Chen, F.; Cai, W. Pharmacokinetic issues of imaging with nanoparticles: Focusing on carbon nanotubes and quantum dots. Mol. Imaging Biol. 2013, 15, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Kim, Y.K.; Shin, D.; Ryoo, S.R.; Hong, B.H.; Min, D.H. Biomedical applications of graphene and graphene oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Barnes, J.C.; Bosoy, A.; Stoddart, J.F.; Zink, J.I. Mesoporous silica nanoparticles in biomedical applications. Chem. Soc. Rev. 2012, 41, 2590–2605. [Google Scholar] [CrossRef] [PubMed]
- Bitar, A.; Ahmad, N.M.; Fessi, H.; Elaissari, A. Silica-based nanoparticles for biomedical applications. Drug Discov. Today 2012, 17, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Soenen, S.J.; Parak, W.J.; Rejman, J.; Manshian, B. (Intra)cellular stability of inorganic nanoparticles: Effects on cytotoxicity, particle functionality, and biomedical applications. Chem. Rev. 2015, 115, 2109–2135. [Google Scholar] [CrossRef] [PubMed]
- Dou, Q.Q.; Guo, H.C.; Ye, E. Near-infrared upconversion nanoparticles for bio-applications. Mater. Sci. Eng. C 2014, 45, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Li, J.; Liu, F.; Padmanabhan, P.; Yeow, E.; Xing, B. Recent advance of biological molecular imaging based on lanthanide-doped upconversion-luminescent nanomaterials. Nanomaterials 2014, 4, 129–154. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Mukherjee, P. Biological properties of “naked” metal nanoparticles. Adv. Drug Deliv. Rev. 2008, 60, 1289–1306. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef] [PubMed]
- Dreaden, E.C.; Alkilany, A.M.; Huang, X.; Murphy, C.J.; El-Sayed, M.A. The golden age: Gold nanoparticles for biomedicine. Chem. Soc. Rev. 2012, 41, 2740–2779. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Vig, K.; Dennis, V.; Singh, S. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.S.; Chen, G. Silver nanoparticles: Synthesis, properties, and therapeutic applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef] [PubMed]
- Ling, D.; Hyeon, T. Chemical design of biocompatible iron oxide nanoparticles for medical applications. Small 2013, 9, 1450–1466. [Google Scholar] [CrossRef] [PubMed]
- Gregoriadis, G. Engineering liposomes for drug delivery: Progress and problems. Trends Biotechnol. 1995, 13, 527–537. [Google Scholar] [CrossRef]
- Lasic, D.D. Novel applications of liposomes. Trends Biotechnol. 1998, 16, 307–321. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, Y. Physicochemical characteristics of nanoparticles affect circulation, biodistribution, cellular internalization, and trafficking. Small 2013, 9, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Jo, D.H.; Kim, J.H.; Lee, T.G.; Kim, J.H. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomedicine 2015, 11, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Y.; Yu, J.C. Chemical modification of inorganic nanostructures for targeted and controlled drug delivery in cancer treatment. J. Mater. Chem. B 2014, 2, 452–470. [Google Scholar] [CrossRef]
- Kobayashi, K.; Wei, J.; Iida, R.; Ijiro, K.; Niikura, K. Surface engineering of nanoparticles for therapeutic applications. Polym. J. 2014, 46, 460–468. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Erathodiyil, N.; Ying, J.Y. Functionalization of inorganic nanoparticles for bioimaging applications. Acc. Chem. Res. 2011, 44, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Liu, J.; Zhao, X.; Zhang, Y.; Liu, J.; Xu, S.; Deng, L.; Dong, A.; Zhang, J. PEG-b-PCL copolymer micelles with the ability of pH-controlled negative-to-positive charge reversal for intracellular delivery of doxorubicin. Biomacromolecules 2014, 15, 4281–4292. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Song, I.; Um, S. Role of physicochemical properties in nanoparticle toxicity. Nanomaterials 2015, 5, 1351–1365. [Google Scholar] [CrossRef]
- Decuzzi, P.; Lee, S.; Bhushan, B.; Ferrari, M. A theoretical model for the margination of particles within blood vessels. Ann. Biomed. Eng. 2005, 33, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, H.; Liu, J.; Deng, L.; Liu, J.; Dong, A.; Zhang, J. Comb-like amphiphilic copolymers bearing acetal-functionalized backbones with the ability of acid-triggered hydrophobic-to-hydrophilic transition as effective nanocarriers for intracellular release of curcumin. Biomacromolecules 2013, 14, 3973–3984. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Li, X.; Wang, Q.; Liao, L.; Zhang, C. Nanocomposite hydrogels and their applications in drug delivery and tissue engineering. J. Biomed. Nanotechnol. 2015, 11, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Usuki, A.; Kojima, Y.; Kawasumi, M.; Okada, A.; Fukushima, Y.; Kurauchi, T.; Kamigaito, O. Mechanical properties of nylon 6-clay hybrid. J. Mater. Res. 1993, 8, 1179–1184. [Google Scholar] [CrossRef]
- Yano, K.; Usuki, A.; Okada, A.; Kurauch, T.; Kamigaito, O. Synthesis and properties of polyimide-clay hybrid. J. Polym. Sci. A Polym. Chem. 1993, 31, 2493–2498. [Google Scholar] [CrossRef]
- Kokabi, M.; Sirousazar, M.; Hassan, Z.M. PVA-clay nanocomposite hydrogels for wound dressing. Eur. Polym. J. 2007, 43, 773–781. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, H.; Konst, S.; Sarmiento, R.; Rajachar, R.; Lee, B.P. Injectable dopamine-modified poly(ethylene glycol) nanocomposite hydrogel with enhanced adhesive property and bioactivity. ACS Appl. Mater. Interfaces 2014, 6, 16982–16992. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Du, X.; Zhang, R.; Deng, L.; Dong, A.; Zhang, J. Bioadhesive film formed from a novel organic-inorganic hybrid gel for transdermal drug delivery system. Eur. J. Pharm. Biopharm. 2011, 79, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Mohan, Y.M.; Lee, K.; Premkumar, T.; Geckeler, K.E. Hydrogel networks as nanoreactors: A novel approach to silver nanoparticles for antibacterial applications. Polymer 2007, 48, 158–164. [Google Scholar] [CrossRef]
- Suvorova, E.I.; Klechkovskaya, V.V.; Kopeikin, V.V.; Buffat, P.A. Stability of Ag nanoparticles dispersed in amphiphilic organic matrix. J. Cryst. Growth 2005, 275, 2351–2356. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, J.; Paquet, C.; Lin, Y.; Kumacheva, E. From hybrid microgels to photonic crystals. Adv. Funct. Mater. 2003, 13, 468–472. [Google Scholar] [CrossRef]
- Sershen, S.R.; Westcott, S.L.; Halas, N.J.; West, J.L. Independent optically addressable nanoparticle-polymer optomechanical composites. Appl. Phys. Lett. 2002, 80, 4609–4611. [Google Scholar] [CrossRef]
- Holtz, J.H.; Asher, S.A. Polymerized colloidal crystal hydrogel films as intelligent chemical sensing materials. Nature 1997, 389, 829–832. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, P.; Sun, Y.; Wu, Y.; Mayers, B.; Gates, B.; Yin, Y.; Kim, F.; Yan, H. One-dimensional nanostructures: Synthesis, characterization, and applications. Adv. Mater. 2003, 15, 353–389. [Google Scholar] [CrossRef]
- Pardo-Yissar, V.; Gabai, R.; Shipway, A.N.; Bourenko, T.; Willner, I. Gold nanoparticle/hydrogel composites with solvent-switchable electronic properties. Adv. Mater. 2001, 13, 1320–1323. [Google Scholar] [CrossRef]
- Sheeney-Haj-Ichia, L.; Sharabi, G.; Willner, I. Control of the electronic properties of thermosensitive poly(N-isopropylacrylamide) and Au-nano-particle/poly(N-isopropylacrylamide) composite hydrogels upon phase transition. Adv. Funct. Mater. 2002, 12, 27–31. [Google Scholar] [CrossRef]
- Jones, C.D.; Lyon, L.A. Photothermal patterning of microgel/gold nanoparticle composite colloidal crystals. J. Am. Chem. Soc. 2003, 125, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Flynn, N.T.; Langer, R. Controlled structure and properties of thermoresponsive nanoparticle-hydrogel composites. Adv. Mater. 2004, 16, 1074–1079. [Google Scholar] [CrossRef]
- Castaneda, L.; Valle, J.; Yang, N.; Pluskat, S.; Slowinska, K. Collagen cross-linking with Au nanoparticles. Biomacromolecules 2008, 9, 3383–3388. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Prevoteau, A.; Elziere, P.; Hourdet, D.; Marcellan, A.; Leibler, L. Nanoparticle solutions as adhesives for gels and biological tissues. Nature 2014, 505, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Yu, G.; Pan, L.; Liu, N.; McDowell, M.T.; Bao, Z.; Cui, Y. Stable Li-ion battery anodes by in-situ polymerization of conducting hydrogel to conformally coat silicon nanoparticles. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Cha, C.; Shin, S.R.; Annabi, N.; Dokmeci, M.R.; Khademhosseini, A. Carbon-Based Nanomaterials: Multifunctional Materials for Biomedical Engineering. ACS Nano 2013, 7, 2891–2897. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Banerjee, A. Short peptide based hydrogels: Incorporation of graphene into the hydrogel. Soft Matter 2011, 7, 9259–9266. [Google Scholar] [CrossRef]
- Shin, S.R.; Hojae, B.; Cha, J.M.; Mun, J.Y.; Chen, Y.C.; Tekin, H.; Shin, H.; Farshch, S.; Dokmeci, M.R.; Tang, S.; et al. Carbon nanotube reinforced hybrid microgels as scaffold materials for cell encapsulation. ACS Nano 2012, 6, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Arslantunali, D.; Budak, G.; Hasirci, V. Multiwalled CNT-pHEMA composite conduit for peripheral nerve repair. J. Biomed. Mater. Res. A 2014, 102, 828–841. [Google Scholar] [CrossRef] [PubMed]
- Davaran, S.; Alimirzalu, S.; Nejati-Koshki, K.; Nasrabadi, H.T.; Akbarzadeh, A.; Khandaghi, A.A.; Abbasian, M.; Alimohammadi, S. Physicochemical characteristics of Fe3O4 magnetic nanocomposites based on poly(N-isopropylacrylamide) for anti-cancer drug delivery. Asian Pac. J. Cancer Prev. 2014, 15, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Evingur, G.A.; Pekcan, O. Effect of multiwalled carbon nanotube (MWNT) on the behavior of swelling of polyacrylamide-MWNT composites. J. Reinf. Plast. Comp. 2014, 33, 1199–1206. [Google Scholar] [CrossRef] [Green Version]
- Curcio, M.; Spizzirri, U.G.; Cirillo, G.; Vittorio, O.; Picci, N.; Nicoletta, F.P.; Iemma, F.; Hampel, S. On demand delivery of ionic drugs from electro-responsive CNT hybrid films. RSC Adv. 2015, 5, 44902–44911. [Google Scholar] [CrossRef]
- Toumia, Y.; Orlanducci, S.; Basoli, F.; Licoccia, S.; Paradossi, G. “Soft” confinement of graphene in hydrogel matrixes. J. Phys. Chem. B 2015, 119, 2051–2061. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, H.; Ramazani, S.A.A.; Mashayekhan, S. Fabrication and characterization of conductive chitosan/gelatin-based scaffolds for nerve tissue engineering. Int. J. Biol. Macromol. 2015, 74, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Pei, X.; Zeng, Y.; He, R.; Li, Z.; Wang, J.; Wan, Q.; Li, X. Functionalized nanoscale graphene oxide for high efficient drug delivery of cisplatin. J. Nanopart. Res. 2014, 16, 1–4. [Google Scholar] [CrossRef]
- Guo, Q.F.; Cao, H.; Li, X.H.; Liu, S.W. Thermosensitive hydrogel drug delivery system containing doxorubicin loaded CS–GO nanocarriers for controlled release drug in situ. Mater. Technol. 2015, 30, 294–300. [Google Scholar] [CrossRef]
- Byrne, M.T.; Guńko, Y.K. Recent advances in research on carbonnanotube-polymer composites. Adv. Mater. 2010, 22, 1672–1688. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, M.; Chen, H.; Xu, J.; Gao, Y.; Li, H. Phenylboronate-diol crosslinked polymer/SWCNT hybrid gels with reversible sol-gel transition. Polym. Adv. Technol. 2014, 25, 233–239. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Faĺko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, A.; Qin, M.; Huang, R.; Zhang, G.; Xue, B.; Wei, J.; Li, Y.; Cao, Y.; Wang, W. Hierarchical construction of a mechanically stable peptide-graphene oxide hybrid hydrogel for drug delivery and pulsatile triggered release in vivo. Nanoscale 2015, 7, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, H.; Vondem-Bussche, A.; Creighton, M.; Hurt, R.H.; Kane, A.B.; Gaoa, H. Graphene microsheets enter cells through spontaneous membrane penetration at edge asperities and corner sites. Proc. Natl. Acad. Sci. USA 2013, 110, 12295–12300. [Google Scholar] [CrossRef] [PubMed]
- Mac Kenna, N.; Calvert, P.; Morrin, A.; Wallacec, G.G.; Moulton, S.E. Electro-stimulated release from a reduced graphene oxide composite hydrogel. J. Mater. Chem. B 2015, 3, 2530–2537. [Google Scholar] [CrossRef]
- Shen, J.; Xu, G.; Xin, X.; Wang, L.; Song, Z.; Zhang, H.; Tong, L.; Yang, Z. Supramolecular hydrogels of α-cyclodextrin/reverse poloxamines/carbon-based nanomaterials and its multi-functional application. RSC Adv. 2015, 5, 40173–40182. [Google Scholar] [CrossRef]
- Luo, H.; Xiong, G.; Yang, Z.; Raman, S.R.; Si, H.; Wan, Y. A novel three-dimensional graphene/bacterial cellulose nanocomposite prepared by in situ biosynthesis. RSC Adv. 2014, 4, 14369–14372. [Google Scholar] [CrossRef]
- Kun, J.; Ye, C.; Zhang, P.; Wang, X.; Zhao, Y. One-pot controlled synthesis of homopolymers and diblock copolymers grafted graphene oxide using couplable raft agents. Macromolecules 2012, 45, 1346–1355. [Google Scholar]
- Mohammad Mahdi Dadfar, S.; Kavoosi, G.; Mohammad Ali Dadfar, S. Investigation of mechanical properties, antibacterial features, and water vapor permeability of polyvinyl alcohol thin films reinforced by glutaraldehyde and multiwalled carbon nanotube. Polym. Compos. 2014, 35, 1736–1743. [Google Scholar] [CrossRef]
- Choi, E.J.; Shin, J.; Khaleel, Z.H.; Cha, I.; Yun, S.-H.; Cho, S.W.; Song, C. Synthesis of electroconductive hydrogel films by an electro-controlled click reaction and their application to drug delivery systems. Polym. Chem. 2015, 6, 4473–4478. [Google Scholar] [CrossRef]
- Wang, P.; Wang, S.; Lian, L.; Liu, Y.; Zhang, M. Preparation and properties of novel microporous hydrogels with poly(ethylene glycol) dimethacrylate and carboxylated carbon nanotubes. J. Control. Release 2015, 213, e86. [Google Scholar] [CrossRef]
- Mu, S.; Liang, Y.; Chen, S.; Zhang, L.; Liu, T. MWNT-hybrided supramolecular hydrogel for hydrophobic camptothecin delivery. Mater. Sci. Eng. C 2015, 50, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.; Bhunia, T.; Goswami, L.; Panda, A.B.; Bandyopadhyay, A. Fabrication of acrylic acid grafted guar gum-multiwalled carbon nanotube hydrophobic membranes for transdermal drug delivery. RSC Adv. 2015, 5, 41736–41744. [Google Scholar] [CrossRef]
- Shi, X.; Zheng, Y.; Wang, C.; Yue, L.; Qiao, K.; Wang, G.; Wang, L.; Quan, H. Dual stimulus responsive drug release under the interaction of pH value and pulsatile electric field for a bacterial cellulose/sodium alginate/multi-walled carbon nanotube hybrid hydrogel. RSC Adv. 2015, 5, 41820–41829. [Google Scholar] [CrossRef]
- Zinchenko, A.; Taki, Y.; Sergeyev, V.; Murata, S. DNA-assisted solubilization of carbon nanotubes and construction of DNA-MWCNT cross-linked hybrid hydrogels. Nanomaterials 2015, 5, 270–283. [Google Scholar] [CrossRef]
- Sun, Y.X.; Ren, K.F.; Chang, G.X.; Zhao, Y.X.; Liu, X.S.; Ji, J. Enhanced electrochemical stimuli multilayers based on a ferrocene-containing polymer. Sci. Bull. 2015, 60, 936–942. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, J.; Sun, H.; Qiu, X.; Mou, Y.; Liu, Z.; Zhao, Y.; Li, X.; Han, Y.; Duan, C.; et al. Engineering the heart: Evaluation of conductive nanomaterials for improving implant integration and cardiac function. Sci. Rep. 2014, 4, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Jayakumar, R.; Mohandas, A.; Bhatnagar, I.; Kim, S.K. Antimicrobial activity of chitosan-carbon nanotube hydrogels. Materials 2014, 7, 3946–3955. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.Y.; Yang, S.H.; Lee, E.J.; Kim, H.W. Carbon nanotube-collagen three-dimensional culture of mesenchymal stem cells promotes expression of neural phenotypes and secretion of neurotrophic factors. Acta Biomater. 2014, 10, 4425–4436. [Google Scholar] [CrossRef] [PubMed]
- Mandal, D.; Kar, T.; Das, P.K. Pyrene-based fluorescent ambidextrous gelators: Scaffolds for mechanically robust SWNT-gel nanocomposites. Chem. Eur. J. 2014, 20, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Sheikholeslam, M.; Pritzker, M.; Chen, P. Hybrid peptide-carbon nanotube dispersions and hydrogels. Carbon 2014, 71, 284–293. [Google Scholar] [CrossRef]
- Tardani, F.; La Mesa, C. Effects of single-walled carbon nanotubes on lysozyme gelation. Colloid Surface B 2014, 121, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cui, L.; Kong, N.; Barrow, C.J.; Yang, W. RAFT controlled synthesis of graphene/polymer hydrogel with enhanced mechanical property for pH-controlled drug release. Eur. Polym. J. 2014, 50, 9–17. [Google Scholar] [CrossRef]
- Servant, A.; Leon, V.; Jasim, D.; Methven, L.; Limousin, P.; Fernandez-Pacheco, E.V.; Prato, M.; Kostarelos, K. Graphene-based electroresponsive scaffolds as polymeric implants for on-demand drug delivery. Adv. Healthc. Mater. 2014, 3, 1334–1343. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wang, X.; Wang, G.Y.; Duo, Y.R.; Zhang, X.Y.; Hu, X.H.; Zhang, X.J. Double network self-healing graphene hydrogel by two step method for anticancer drug delivery. Mater. Technol. 2014, 29, 210–213. [Google Scholar] [CrossRef]
- Faghihi, S.; Karimi, A.; Jamadi, M.; Imani, R.; Salarian, R. Graphene oxide/poly(acrylic acid)/gelatin nanocomposite hydrogel: Experimental and numerical validation of hyperelastic model. Mater. Sci. Eng. C 2014, 38, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.; Chen, B. Self-assembled graphene oxide-gelatin nanocomposite hydrogels: Characterization, formation mechanisms, and pH-sensitive drug release behavior. J. Polym. Sci. Polym. Phys. 2015, 53, 356–367. [Google Scholar] [CrossRef]
- Shen, J.; Xin, X.; Zhang, Y.; Song, L.; Wang, L.; Tang, W.; Ren, Y. Manipulation the behavior of supramolecular hydrogels of alpha-cyclodextrin/star-like block copolymer/carbon-based nanomaterials. Carbohydr. Polym. 2015, 117, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, C.; Shuai, Y.; Cui, X.; Nie, L. Controlled release of anticancer drug using graphene oxide as a drug-binding effector in konjac glucomannan/sodium alginate hydrogels. Colloid. Surface. B 2014, 113, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Han, L.; Tang, Y.; Ren, J.; Zhao, Z.; Jia, L. Highly flexible heparin-modified chitosan/graphene oxide hybrid hydrogel as a super bilirubin adsorbent with excellent hemocompatibility. J. Mater. Chem. B 2015, 3, 1646–1654. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, T.; Zhao, L.; Sun, W.; Liu, X.; Tong, Z. Fast self-healing of graphene oxide-hectorite clay-poly(N,N-dimethylacrylamide) hybrid hydrogels realized by near-infrared irradiation. ACS Appl. Mater. Interfaces 2014, 6, 22855–22861. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, R.; Bag, D.S.; Nigam, I. Fullerene (C60) Containing Poly(N-isopropylacrylamide) Thermo-responsive Smart Hydrogels and their Swelling Behavior. J. Polym. Mater. 2013, 30, 15–26. [Google Scholar]
- Katiyar, R.; Bag, D.S.; Nigam, I. Synthesis and evaluation of swelling characteristics of fullerene (C60) containing cross-linked poly(2-hydroxyethyl methacrylate) hydrogels. Adv. Mater. Lett. 2014, 5, 214–222. [Google Scholar]
- Kim, S.; Lee, D.J.; Kwag, D.S.; Lee, U.Y.; Youn, Y.S.; Lee, E.S. Acid pH-activated glycol chitosan/fullerene nanogels for efficient tumor therapy. Carbohydr. Polym. 2014, 101, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glassceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Markel, D.C.; Jin, X.; Shi, T.; Ren, W. Poly(vinyl alcohol)/collagen/hydroxyapatite hydrogel: Properties and in vitro cellular response. J. Biomed. Mater. Res. A 2012, 100, 3071–3079. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Mihaila, S.M.; Swami, A.; Patel, A.; Sant, S.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Bioactive silicate nanoplatelets for osteogenic diffrentiation of human human mesenchymal stem cells. Adv. Mater. 2013, 25, 3329–3336. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Weir, M.D.; Xu, H.H. An injectable calcium phosphate-alginate hydrogelumbilical cord mesenchymal stem cell paste for bone tissue engineering. Biomaterials 2010, 31, 6502–6510. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; El-Fiqi, A.; Han, C.M.; Kim, H.W. Physically-strengthened collagen bioactive nanocomposite gels for bone: A feasibility study. Tissue Eng. Regen. Med. 2015, 12, 90–97. [Google Scholar] [CrossRef]
- Aguzzi, C.; Capra, P.; Bonferoni, C.; Cerezo, P.; Salcedo, I.; Sánchez, R.; Caramella, C.; Viseras, C. Chitosan-silicate biocomposites to be used in modified drug release of 5-aminosalicylic acid (5-ASA). Appl. Clay Sci. 2010, 50, 106–111. [Google Scholar] [CrossRef]
- Kawachi, Y.; Kugimiya, S.; Kato, K. Preparation of silica hydrogels using a synthetic peptide for application as carriers for controlled drug release and mesoporous oxides. J. Ceram. Soc. Jpn. 2014, 122, 134–140. [Google Scholar] [CrossRef]
- Karnik, S.; Hines, K.; Mills, D.K. Nanoenhanced hydrogel system with sustained release capabilities. J. Biomed. Mater. Res. A 2015, 103, 2416–2426. [Google Scholar] [CrossRef] [PubMed]
- Ayyub, O.B.; Kofinas, P. Enzyme Induced stiffening of nanoparticle-hydrogel composites with structural color. ACS Nano 2015, 9, 8004–8011. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, X.; Wang, K.; Wang, Q.; Ji, H.; Wu, C.; Li, J.; He, X.; Tang, J.; Huang, J. Combining physical embedding and covalent bonding for stable encapsulation of quantum dots into agarose hydrogels. J. Mater. Chem. 2012, 22, 495–501. [Google Scholar] [CrossRef]
- Yuan, J.; Wen, D.; Gaponik, N.; Eychmuller, A. Enzyme-encapsulating quantum dot hydrogels and xerogels as biosensors: Multifunctional platforms for both biocatalysis and fluorescent probing. Angew. Chem. Int. Ed. Engl. 2013, 52, 976–979. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Zhang, J.; Huang, L.; Liu, J.; Li, Y.; Zhang, G.; Kundu, S.C.; Wang, L. Exploring natural silk protein sericin for regenerative medicine: An injectable, photoluminescent, cell-adhesive 3D hydrogel. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hong, X.; Liu, Y.; Li, D.; Wang, Y.W.; Li, J.H.; Bai, Y.B.; Li, T.J. Highly photoluminescent cdte/poly(n-isopropylacrylamide) temperature-sensitive gels. Adv. Mater. 2005, 17, 163–166. [Google Scholar] [CrossRef]
- Amdursky, N.; Gazit, E.; Rosenman, G. Quantum confinement in self-assembled bioinspired peptide hydrogels. Adv. Mater. 2010, 22, 2311–2315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yang, J.; Bao, S.; Wu, Q.; Wang, Q. Semiconductor nanoparticle-based hydrogels prepared via self-initiated polymerization under sunlight, even visible light. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Jiang, M. Supramolecular hydrogels with CdS quantum dots incorporated by host-guest interactions. Macromol. Rapid Commun. 2010, 31, 1736–1739. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.S.; Li, C.M. Quantum dot-based nanocomposites for biomedical applications. Curr. Med. Chem. 2011, 18, 3516–3528. [Google Scholar] [CrossRef] [PubMed]
- Biju, V.; Itoh, T.; Ishikawa, M. Delivering quantum dots to cells: Bioconjugated quantum dots for targeted and nonspecific extracellular and intracellular imaging. Chem. Soc. Rev. 2010, 39, 3031–3056. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, F. Thermo-sensitive and photoluminescent hydrogels: Synthesis, characterization, and their drug-release property. Mater. Sci. Eng. C Mater. Biol. Appl. 2011, 31, 1429–1435. [Google Scholar] [CrossRef]
- Kim, J.H.; Lim, S.Y.; Nam, D.H.; Ryu, J.; Ku, S.H.; Park, C.B. Self-assembled, photoluminescent peptide hydrogel as a versatile platform for enzyme-based optical biosensors. Biosens. Bioelectron. 2011, 26, 1860–1865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; He, X.W.; Li, W.Y.; Zhang, Y.K. Thermo-sensitive imprinted polymer coating CdTe quantum dots for target protein specific recognition. Chem. Commun. 2012, 48, 1757–1759. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Huang, L.; Lin, Y.; Shen, L.; Chen, Q.; Shi, W. Printable temperature-responsive hybrid hydrogels with photoluminescent carbon nanodots. Nanotechnology 2014, 25, 055603. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Chen, L.; Wang, C.-F.; Chen, S. Facile synthesis of fluorescent quantum dot-polymer nanocomposites via frontal polymerization. J. Polym. Sci. A Polym. Chem. 2010, 48, 2170–2177. [Google Scholar] [CrossRef]
- Gattas-Asfura, K.M.; Zheng, Y.; Micic, M.; Snedaker, M.J.; Ji, X.; Sui, G.; Orbulescu, J.; Andreopoulos, F.M.; Pham, S.M.; Wang, C.; et al. Immobilization of quantum dots in the photo-cross-linked poly(ethylene glycol)-based hydrogel. J. Phys. Chem. B 2003, 107, 10464–10469. [Google Scholar] [CrossRef]
- Xie, X.; Ma, D.; Zhang, L.-M. Fabrication and properties of a supramolecular hybrid hydrogel doped with CdTe quantum dots. RSC Adv. 2015, 5, 58746–58754. [Google Scholar] [CrossRef]
- Gong, Y.; Gao, M.; Wang, D.; Möhwald, H. Incorporating fluorescent CdTe nanocrystals into a hydrogel via hydrogen bonding: Toward fluorescent microspheres with temperature-responsive properties. Chem. Mater. 2005, 17, 2648–2653. [Google Scholar] [CrossRef]
- Bai, S.; Nguyen, T.L.; Mulvaney, P.; Wang, D. Using hydrogels to accommodate hydrophobic nanoparticles in aqueous media via solvent exchange. Adv. Mater. 2010, 22, 3247–3250. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, R.; Xu, S.; Liu, H.; Hu, Y. Thermoresponsive behavior and rheology of SiO2-hyaluronic acid/poly(N-isopropylacrylamide) (NaHA/PNIPAm) core-shell structured microparticles. J. Chem. Technol. Biotechnol. 2015, 90, 407–414. [Google Scholar] [CrossRef]
- Dogru, O.; Abdurrahmanoglu, S.; Kayaman-Apohan, N. Preparation and characterization of modified nanosilica/PNIPAm hybrid cryogels. Polym. Bull. 2015, 72, 993–1005. [Google Scholar] [CrossRef]
- Garnweitner, G.; Smarsly, B.; Assink, R.; Dunphy, D.R.; Scullin, C.; Brinker, C.J. Characterization of self-assembled lamellar thermoresponsive silica-hydrogel nanocomposite films. Langmuir 2004, 20, 9811–9820. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.W.; Kim, H.I. Preparation of temperature-responsive hydrogel nanocapsules with interfacial modification of colloidal silica. Compos. Interfaces 2006, 13, 277–284. [Google Scholar] [CrossRef]
- Kurumada, K.I.; Nakamura, T.; Suzuki, A.; Umeda, N.; Kishimoto, N.; Hiro, M. Nanoscopic replication of cross-linked hydrogel in high-porosity nanoporous silica. J. Non-Cryst. Solids 2007, 353, 4839–4844. [Google Scholar] [CrossRef]
- Van den Brom, C.R.; Anac, I.; Roskamp, R.F.; Retsch, M.; Jonas, U.; Menges, B.; Preece, J.A. The swelling behaviour of thermoresponsive hydrogel/silica nanoparticle composites. J. Mater. Chem. 2010, 20, 4827–4839. [Google Scholar] [CrossRef]
- Alam, M.A.; Takafuji, M.; Ihara, H. Thermosensitive hybrid hydrogels with silica nanoparticle-cross-linked polymer networks. J. Colloid Interface Sci. 2013, 405, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Ham, M.J.; Kim, Y.H. Property Modulation of poly(N-isopropylacrylamide) hydrogels by incorporation of modified silica. Polym. Eng. Sci. 2008, 48, 2439–2445. [Google Scholar] [CrossRef]
- Van Durme, K.; van Mele, B.; Loos, W.; Du Prez, F.E. Introduction of silica into thermo-responsive poly(N-isopropyl acrylamide) hydrogels: A novel approach to improve response rates. Polymer 2005, 46, 9851–9862. [Google Scholar] [CrossRef]
- Zaragoza, J.; Babhadiashar, N.; O’Brien, V.; Chang, A.; Blanco, M.; Zabalegui, A.; Lee, H.; Asuri, P. Experimental investigation of mechanical and thermal properties of silica nanoparticle-reinforced poly(acrylamide) nanocomposite hydrogels. PLoS ONE 2015, 10, e0136293. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zeng, L.; Chen, H.; Zhang, C. Effects of silica sol content on the properties of poly(acrylamide)/silica composite hydrogel. Polym. Bull. 2012, 68, 309–316. [Google Scholar] [CrossRef]
- Wang, Q.; Hou, R.; Cheng, Y.; Fu, J. Super-tough double-network hydrogels reinforced by covalently compositing with silica-nanoparticles. Soft Matter 2012, 8, 6048–6056. [Google Scholar] [CrossRef]
- Yang, J.; Deng, L.-H.; Han, C.-R.; Duan, J.-F.; Ma, M.-G.; Zhang, X.-M.; Xu, F.; Sun, R.-C. Synthetic and viscoelastic behaviors of silica nanoparticle reinforced poly(acrylamide) core-shell nanocomposite hydrogels. Soft Matter 2013, 9, 1220–1230. [Google Scholar] [CrossRef]
- Yang, J.; Han, C.R. Dynamics of Silica-nanoparticle-filled hybrid hydrogels: Nonlinear viscoelastic behavior and chain entanglement network. J. Phys. Chem. C 2013, 117, 20236–20243. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, J.J.; Han, C.R.; Duan, J.F. Keys to enhancing mechanical properties of silica nanoparticle composites hydrogels: The role of network structure and interfacial interactions. Compos. Sci. Technol. 2014, 95, 1–7. [Google Scholar] [CrossRef]
- Yin, P.; Huang, G.B.; Tse, W.H.; Bao, Y.G.; Denstedt, J.; Zhang, J. Nanocomposited silicone hydrogels with a laser-assisted surface modification for inhibiting the growth of bacterial biofilm. J. Mater. Chem. B 2015, 3, 3234–3241. [Google Scholar] [CrossRef]
- Lin, M.; Xu, P.; Zhong, W. Preparation, characterization, and release behavior of aspirin-loaded poly(2-hydroxyethyl acrylate)/silica hydrogels. J. Biomed. Mater. Res. B 2012, 100, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ye, L. Structure and property of polyvinyl alcohol/precipitated silica composite hydrogels for microorganism immobilization. Compos. B 2014, 56, 749–755. [Google Scholar] [CrossRef]
- Lewandowska-Lancucka, J.; Fiejdasz, S.; Rodzik, L.; Koziel, M.; Nowakowska, M. Bioactive hydrogel-nanosilica hybrid materials: A potential injectable scaffold for bone tissue engineering. Biomed. Mater. 2015, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhu, Y.; Zhang, L.; Shi, J. Preparation of chitosan/mesoporous silica nanoparticle composite hydrogels for sustained co-delivery of biomacromolecules and small chemical drugs. Sci. Technol. Adv. Mater. 2013, 14, 045005. [Google Scholar] [CrossRef]
- Lou, Y.R.; Kanninen, L.; Kaehr, B.; Townson, J.L.; Niklander, J.; Harjumaki, R.; Jeffrey Brinker, C.; Yliperttula, M. Silica bioreplication preserves three-dimensional spheroid structures of human pluripotent stem cells and HepG2 cells. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Panic, V.V.; Spasojevic, P.M.; Radoman, T.S.; Dzunuzovic, E.S.; Popovic, I.G.; Velickovic, S.J. Methacrylic acid based polymer networks with a high content of unfunctionalized nanosilica: Particle distribution, swelling, and rheological properties. J. Phys. Chem. C 2015, 119, 610–622. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, J. Preparation and mechanical properties of silica nanoparticles reinforced composite hydrogels. Mater. Lett. 2014, 120, 36–38. [Google Scholar] [CrossRef]
- Schlossmacher, U.; Schroeder, H.C.; Wang, X.; Feng, Q.; Diehl-Seifert, B.; Neumann, S.; Trautwein, A.; Mueller, W.E.G. Alginate/silica composite hydrogel as a potential morphogenetically active scaffold for three-dimensional tissue engineering. RSC Adv. 2013, 3, 11185–11194. [Google Scholar] [CrossRef]
- Lucia Foglia, M.; Edhit Camporotondi, D.; Solange Alvarez, G.; Heinemann, S.; Hanke, T.; Javier Perez, C.; Eduardo Diaz, L.; Federico Desimone, M. A new method for the preparation of biocompatible silica coated-collagen hydrogels. J. Mater. Chem. B 2013, 1, 6283–6290. [Google Scholar] [CrossRef] [Green Version]
- Solange Alvarez, G.; Helary, C.; Mathilde Mebert, A.; Wang, X.; Coradin, T.; Federico Desimone, M. Antibiotic-loaded silica nanoparticle-collagen composite hydrogels with prolonged antimicrobial activity for wound infection prevention. J. Mater. Chem. B 2014, 2, 4660–4670. [Google Scholar] [CrossRef]
- Lu, C.; Zahedi, P.; Forman, A.; Allen, C. Multi-arm PEG/silica hydrogel for sustained ocular drug delivery. J. Pharm. Sci. 2014, 103, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Giray, S.; Bal, T.; Kartal, A.M.; Kizilel, S.; Erkey, C. Controlled drug delivery through a novel PEG hydrogel encapsulated silica aerogel system. J. Biomed. Mater. Res. A 2012, 100, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Liu, H.; Deng, H.; Xiao, L.; Qin, C.; Du, Y.; Shi, X. A study of chitosan hydrogel with embedded mesoporous silica nanoparticles loaded by ibuprofen as a dual stimuli-responsive drug release system for surface coating of titanium implants. Colloid. Surface. B 2014, 123, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.C.; Urbano, B.F.; Campos, C.H.; Rivas, B.L.; Reyes, P. Composite hydrogel based on surface modified mesoporous silica and poly[(2-acryloyloxy)ethyl trimethylammonium chloride]. Mater. Chem. Phys. 2015, 152, 69–76. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Nie, W.; Song, L.; Chen, P. Formation and surface properties of raspberry-like silica particles: Effect of molecular weight of the coating poly(methacrylic acid) brushes. J. Sol-Gel Sci. Technol. 2014, 72, 122–129. [Google Scholar] [CrossRef]
- Miyamoto, N.; Shimasaki, K.; Yamamoto, K.; Shintate, M.; Kamachi, Y.; Bastakoti, B.P.; Suzuki, N.; Motokawa, R.; Yamauchi, Y. Mesoporous silica particles as topologically crosslinking fillers for poly(N-isopropylacrylamide) hydrogels. Chemistry 2014, 20, 14955–14958. [Google Scholar] [CrossRef] [PubMed]
- Sadat-Shojai, M.; Khorasani, M.T.; Jamshidi, A. 3-Dimensional cell-laden nano-hydroxyapatite/protein hydrogels for bone regeneration applications. Mater. Sci. Eng. C 2015, 49, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Liu, X.; Wei, D.; Sun, J.; Xiao, W.; Zhao, H.; Guo, L.; Wei, Q.; Fan, H.; Zhang, X. Photo-crosslinkable methacrylated gelatin and hydroxyapatite hybrid hydrogel for modularly engineering biomimetic osteon. ACS Appl. Mater. Interfaces 2015, 7, 10386–10394. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.; Moztarzadeh, F.; Rabiee, M.; Azami, M.; Maleknia, S.; Tahriri, M.; Moztarzadeh, Z.; Nezafati, N. Development of macroporous nanocomposite scaffolds of gelatin/bioactive glass prepared through layer solvent casting combined with lamination technique for bone tisue engineering. Ceram. Int. 2010, 36, 2431–2439. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, G.; Zheng, F.; Han, J.; Wu, X. Novel thermo-sensitive hydrogels containing polythioether dendrons: Facile tuning of LCSTs, strong absorption of Ag ions, and embedment of smaller Ag nanocrystals. Polym. Chem. 2015, 6, 625–632. [Google Scholar] [CrossRef]
- Ribeiro, M.; de Moraes, M.A.; Beppu, M.M.; Garcia, M.P.; Fernandes, M.H.; Monteiro, F.J.; Ferraz, M.P. Development of silk fibroin/nanohydroxyapatite composite hydrogels for bone tissue engineering. Eur. Polym. J. 2015, 67, 66–77. [Google Scholar] [CrossRef]
- Yang, M.; He, W.; Shuai, Y.; Min, S.; Zhu, L. Nucleation of hydroxyapatite crystalsby self-assembled Bombyx mori silk fibroin. J. Polym. Sci. B 2013, 51, 742–748. [Google Scholar] [CrossRef]
- Shi, P.; Teh, T.K.; Toh, S.L.; Goh, J.C. Variation of the effect of calcium phosphateenhancement of implanted silk fibroin ligament bone integration. Biomaterials 2013, 34, 5947–5957. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, T.; Kawashit, M.; Kikuta, K.; Ohtsuki, C. Biomimetic mineralization of calcium phosphate crystals in polyacrylamide hydrogel: Effect of concentrations of calcium and phosphate ions on crystalline phases and morphology. Mater. Sci. Eng. C 2010, 30, 154–159. [Google Scholar] [CrossRef]
- Dhivya, S.; Saravanan, S.; Sastry, T.P.; Selvamurugan, N. Nanohydroxyapatite-reinforced chitosan composite hydrogel for bone tissue repair in vitro and in vivo. J. Nanobiotechnol. 2015, 13. [Google Scholar] [CrossRef] [PubMed]
- Caridade, S.G.; Merino, E.G.; Alves, N.M.; Bermudez Vde, Z.; Boccaccini, A.R.; Mano, J.F. Chitosan membranes containing micro or nano-size bioactive glass particles: Evolution of biomineralization followed by in situ dynamic mechanical analysis. J. Mech. Behav. Biomed. Mater. 2013, 20, 173–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couto, D.S.; Hong, Z.; Mano, J.F. Development of bioactive and biodegradable chitosan-based injectable systems containing bioactive glass nanoparticles. Acta Biomater. 2009, 5, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Couto, D.S.; lves, N.M.; Mano, J.F. Nanostructured multilayer coatings combining chitosan with bioactive glass nanoparticles. J. Nanosci. Nanotechnol. 2009, 9, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Iwatsubo, T.; Kishi, R.; Miura, T.; Ohzono, T.; Yamaguchi, T. Formation of Hydroxyapatite Skeletal Materials from Hydrogel Matrices via Artificial Biomineralization. J. Phys. Chem. B 2015, 119, 8793–8799. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, T.; Kishida, A.; Akashi, M. Hydroxyapatite formation on/in poly(vinyl alcohol) hydrogel matrices using a novel alternate soaking process. Chem. Lett. 1998, 8, 711–712. [Google Scholar] [CrossRef]
- Arora, A.; Sharma, P.; Katti, D.S. Pullulan-based composite scaffolds for bone tissue engineering: Improved osteoconductivity by pore wall mineralization. Carbohydr. Polym. 2015, 123, 180–189. [Google Scholar]
- Fu, S.Z.; Guo, G.; Gong, C.Y.; Zeng, S.; Liang, H.; Luo, F.; Zhang, X.N.; Zhao, X.; Wei, Y.Q.; Qian, Z.Y. Injectable biodegradable thermosensitive hydrogel composite for orthopedic tissue engineering 1: Preparation and characterization of nanohydroxyapatite/poly(ethylene glycol)-poly(ε-caprolactone)-poly(ethylene glycol) hydrogel nanocomposites. J. Phys. Chem. B 2009, 113, 16518–16525. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Sasaki, M.; Taguchi, T. Enhanced ALP activity of MG63 cells cultured on hydroxyapatite-poly(ethylene glycol) hydrogel composites prepared using EDTA-OH. Biomed. Mater. 2015, 10, 015025. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Dammu, S.A.; Canter, J.M.; Wu, C.J.; Schmidt, G. Highly extensible, tough, and elastomeric nanocomposite hydrogels from poly(ethylene glycol) and hydroxyapatite nanoparticles. Biomacromolecules 2011, 12, 1641–1650. [Google Scholar] [CrossRef] [PubMed]
- Rottensteiner, U.; Sarker, B.; Heusinger, D.; Dafinova, D.; Rath, S.; Beier, J.; Kneser, U.; Horch, R.; Detsch, R.; Boccaccini, A.; et al. In vitro and in vivo biocompatibility of alginate dialdehyde/gelatin hydrogels with and without nanoscaled bioactive glass for bone tissue engineering applications. Materials 2014, 7, 1957–1974. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Tolba, E.; Schroder, H.C.; Neufurth, M.; Feng, Q.; Diehl-Seifert, B.; Muller, W.E. Effect of bioglass on growth and biomineralization of SaOS-2 cells in hydrogel after 3D cell bioprinting. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela, F.; Covarrubias, C.; Martinez, C.; Smith, P.; Diaz-Dosque, M.; Yazdani-Pedram, M. Preparation and bioactive properties of novel bone-repair bionanocomposites based on hydroxyapatite and bioactive glass nanoparticles. J. Biomed. Mater. Res. B 2012, 100, 1672–1682. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Guo, Q.; Shores, L.S.; Aly, A.; Ramakrishnan, M.; Kim, G.H.; Lu, Q.; Su, L.; Elisseeff, J.H. Use of a chondroitin sulfate bioadhesive to enhance integration of bioglass particles for repairing critical-size bone defects. J. Biomed. Mater. Res. A 2015, 103, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Eglin, D.; Maalheem, S.; Livage, J.; Coradin, T. In vitro apatite forming ability of type I collagen hydrogels containing bioactive glass and silica sol-gel particles. J. Mater. Sci. Mater. Med. 2006, 17, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, J.P.; Plunkett, N.A.; O’Brien, F.J. Addition of hydroxyapatite improves stiffness, interconnectivity and osteogenic potential of a highly porous collagen-based scaffold for bone tissue regeneration. Eur. Cell Mater. 2010, 20, 218–230. [Google Scholar] [PubMed]
- Marelli, B.; Ghezzi, C.E.; Mohn, D.; Stark, W.J.; Barralet, J.E.; Boccaccini, A.R.; Nazhat, S.N. Accelerated mineralization of dense collagen-nano bioactive glass hybrid gels increases scaffold stiffness and regulates osteoblastic function. Biomaterials 2011, 32, 8915–8926. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Mu, C.; Lin, W.; Ngai, T. Gelatin Effects on the Physicochemical and hemocompatible properties of gelatin/pAAm/laponite nanocomposite hydrogels. ACS Appl. Mater. Interfaces 2015, 7, 18732–18741. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.G.; Purwada, A.; Cerchietti, L.; Inghirami, G.; Melnick, A.; Gaharwar, A.K.; Singh, A. Microscale bioadhesive hydrogel arrays for cell engineering applications. Cell. Mol. Bioeng. 2014, 7, 394–408. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, J.; Li, C.; Lin, M.Y.; Schwarz, S.A.; Rafailovich, M.H.; Sokojov, J. Effect of temperature on shear-induced anisotropic structure in polymer clay hydrogels. Macromol. Rapid Commun. 2006, 27, 1787–1791. [Google Scholar] [CrossRef]
- Chang, C.W.; Spreeuwel, A.V.; Zhang, C.; Varghese, S. PEG/clay nanocomposite hydrogel: A mechanically robust tissue engineering scaffold. Soft Matter 2010, 6, 5157–5164. [Google Scholar] [CrossRef]
- Ding, X.; Vegesna, G.K.; Meng, H.; Winter, A.; Lee, B.P. Nitro-group functionalization of dopamine and its contribution to the viscoelastic properties of catechol-containing nanocomposite hydrogels. Macromol. Chem. Phys. 2015, 216, 1109–1119. [Google Scholar] [CrossRef]
- Li, P.; Kim, N.H.; Lee, J.H. Swelling behavior of polyacrylamide/laponite clay nanocomposite hydrogels: pH-sensitive property. Compos. B 2009, 40, 275–283. [Google Scholar] [CrossRef]
- Okay, O.; Oppermann, W. Polyacrylamide-clay nanocomposite hydrogels: Rheological and light scattering characterization. Macromolecules 2007, 40, 3378–3387. [Google Scholar] [CrossRef]
- Xia, M.; Wu, W.; Liu, F.; Theato, P.; Zhu, M. Swelling behavior of thermosensitive nanocomposite hydrogels composed of oligo(ethylene glycol) methacrylates and clay. Eur. Polym. J. 2015, 69, 472–482. [Google Scholar] [CrossRef]
- Tan, Y.; Wu, R.; Li, H.; Ren, W.; Du, J.; Xu, S.; Wang, J. Electric field-induced gradient strength in nanocomposite hydrogel through gradient crosslinking of clay. J. Mater. Chem. B 2015, 3, 4426–4430. [Google Scholar] [CrossRef]
- Wang, J.; Lin, L.; Cheng, Q.F.; Jiang, L. A strong bio-inspired layered PNIPAM-clay nanocomposite hydrogel. Angew. Chem. Int. Ed. 2012, 51, 4676–4678. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, K.; Li, H.J.; Matsuda, K.; Takehisa, T.; Elliott, E. Mechanism of forming organic/inorganic network structures during in-situ free-radical polymerization in PNIPA-clay nanocomposite hydrogels. Macromolecules 2005, 38, 3482–3490. [Google Scholar] [CrossRef]
- Haraguchi, K.; Li, H.J.; Wu, C.J.; Gaharwar, A.K.; Chan, B.K.; Schmidt, G. Mechanically tough pluronic F127/laponite nanocomposite hydrogels from covalently and physically cross-linked networks. Macromolecules 2011, 44, 8215–8224. [Google Scholar]
- Wu, L.; Ohtani, M.; Tamesue, S.; Ishida, Y.; Aida, T. High water content clay-nanocomposite hydrogels incorporating guanidinium-pendant methacrylamide: Tuning of mechanical and swelling properties by supramolecular approach. J. Polym. Sci. A 2014, 52, 839–847. [Google Scholar] [CrossRef]
- Neufeld, L.; Bianco-Peled, H. Designing a biocompatible hydrogel for the delivery of mesalamine. Int. J. Pharm. 2015, 491, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Ferfera Harrar, H.; Aiouaz, N.; Dairi, N.; Hadj-Hamou, A.S. Preparation of chitosan-g-poly (acrylamide)/montmorillonite superabsorbent polymer composites: Studies on swelling, thermal, and antibacterial properties. J. Appl. Polym. Sci. 2014, 131, 39747–39761. [Google Scholar] [CrossRef]
- Dadkhah, D.; Navarchian, A.H.; Aref, L.; Tavakoli, N. Application of taguchi method to investigate the drug release behavior of poly(acrylamide-co-maleic acid)/montmorillonite nanocomposite hydrogels. Adv. Polym. Technol. 2014, 33. [Google Scholar] [CrossRef]
- Ibraeva, Z.E.; Zhumaly, A.A.; Blagih, E.; Kudaibergenov, S.E. Preparation and characterization of organic-inorganic composite materials based on poly(acrylamide) hydrogels and clay minerals. Macromol. Symp. 2015, 351, 97–111. [Google Scholar] [CrossRef]
- Salimi, F.; Sefti, M.V.; Jarrahian, K.; Rafipoor, M.; Ghorashi, S.S. Preparation and investigation of the physical and chemical properties of clay-based polyacrylamide/Cr (III) hydrogels as a water shut-off agent in oil reservoirs. Korean J. Chem. Eng. 2014, 31, 986–993. [Google Scholar] [CrossRef]
- Li, T.; Xiang, S.; Ma, P.; Bai, H.; Dong, W.; Chen, M. Nanocomposite hydrogel consisting of Na-montmorillonite with enhanced mechanical properties. J. Polym. Sci. B Polym. Phys. 2015, 53, 1020–1026. [Google Scholar] [CrossRef]
- Kevadiya, B.D.; Rajkumar, S.; Bajaj, H.C.; Chettiar, S.S.; Gosai, K.; Brahmbhatt, H.; Bhatt, A.S.; Barvaliya, Y.K.; Dave, G.S.; Kothari, R.K. Biodegradable gelatin-ciprofloxacin-montmorillonite composite hydrogels for controlled drug release and wound dressing application. Colloid. Surface. B 2014, 122, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Pour-Esmaeil, S.; Taheri Qazvini, N.; Mahdavi, H. Interpenetrating polymer networks (IPN) based on gelatin/poly(ethylene glycol) dimethacrylate/clay nanocomposites: Structure-properties relationship. Mater. Chem. Phys. 2014, 143, 1396–1403. [Google Scholar] [CrossRef]
- Pavlukhina, S.; Zhuk, I.; Mentbayeva, A.; Rautenberg, E.; Chang, W.; Yu, X.; van de Belt-Gritter, B.; Busscher, H.J.; van der Mei, H.C.; Sukhishvili, S.A. Small-molecule-hosting nanocomposite films with multiple bacteria-triggered responses. NPG Asia Mater. 2014, 6, e121. [Google Scholar] [CrossRef]
- Zhang, S.; Guan, Y.; Fu, G.-Q.; Chen, B.-Y.; Peng, F.; Yao, C.-L.; Sun, R.-C. Organic/inorganic superabsorbent hydrogels based on xylan and montmorillonite. J. Nanomater. 2014, 2014, 2. [Google Scholar] [CrossRef]
- Nistor, M.T.; Vasile, C.; Chiriac, A.P. Hybrid collagen-based hydrogels with embedded montmorillonite nanoparticles. Mater. Sci. Eng. C 2015, 53, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, K.; Takehisa, T.; Fan, S. Effects of clay content on the properties of nanocomposite hydrogels composed of poly(N-isopropylacrylamide) and clay. Macromolecules 2002, 35, 10162–10171. [Google Scholar] [CrossRef]
- Ma, R.-F.; Yuan, J.; Li, C.-C.; Peng, J.; Dung, Z.; Xu, L.; Li, J.-Q.; Zhai, M.-L. Radiation synthesis of polymer/clay nanocomposite hydrogels with high mechanical strength. Nucl. Sci. Tech. 2014, 25, 060301. [Google Scholar]
- Stempfle, B.; Grosse, A.; Ferse, B.; Arndt, K.-F.; Woell, D. Anomalous diffusion in thermoresponsive polymer-clay composite hydrogels probed by wide-field fluorescence microscopy. Langmuir 2014, 30, 14056–14061. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Liu, Z.; Yang, C.; Wang, W.; Ju, X.-J.; Xie, R.; Chu, L.-Y. Poly(N-isopropylacrylamide)-clay nanocomposite hydrogels with responsive bending property as temperature-controlled manipulators. Adv. Funct. Mater. 2015, 25, 2980–2991. [Google Scholar] [CrossRef]
- Wan, T.; Cheng, W.; Zhou, Z.; Xu, M.; Zou, C.; Li, R. Influence of crosslinker amount on swelling and gel properties of hectorite/poly(acrylamide/itaconic acid) nanocomposite hydrogels. Korean J. Chem. Eng. 2015, 32, 1434–1439. [Google Scholar] [CrossRef]
- Wan, T.; Zou, C.; Wang, L.; Wu, D.; Cheng, W.; Li, R.; Xu, M. Hectorite effects on swelling and gel properties of hectorite/poly(AM/IA) nanocomposite hydrogels. Polym. Bull. 2015, 72, 1113–1125. [Google Scholar] [CrossRef]
- Wang, L.A.N.; Cheng, W.; Wan, T.A.O.; Hu, Z.; Xu, M.I.N.; Li, R.; Zou, C. Synthesis and properties of hectorite/poly(AM/IA) nanocomposite hydrogels with high gel strength. J. Chem. Sci. 2015, 127, 19–23. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, N.C. Synthesis of a novel pH responsive phyllosilicate loaded polymeric hydrogel based on poly(acrylic acid-co-N-vinylpyrrolidone) and polyethylene glycol for drug delivery: Modelling and kinetics study for the sustained release of an antibiotic drug. RSC Adv. 2015, 5, 18312–18327. [Google Scholar] [CrossRef]
- Niu, Y.; Dong, W.; Guo, H.; Deng, Y.; Guo, L.; An, X.; He, D.; Wei, J.; Li, M. Mesoporous magnesium silicate-incorporated poly(epsilon-caprolactone)-poly(ethylene glycol)-poly(epsilon-caprolactone) bioactive composite beneficial to osteoblast behaviors. Int. J. Nanomed. 2014, 9, 2665–2675. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Du, G.; Cheng, Y.; Fu, J. Tough nanocomposite double network hydrogels reinforced with clay nanorods through covalent bonding and reversible chain adsorption. J. Mater. Chem. B 2014, 2, 1539–1548. [Google Scholar] [CrossRef]
- Shikinaka, K.; Yokoi, T.; Koizumi-Fujii, Y.; Shimotsuya, M.; Shigehara, K. Robust imogolite hydrogels with tunable physical properties. RSC Adv. 2015, 5, 46493–46500. [Google Scholar] [CrossRef]
- Schexnailder, P.; Schmidt, G. Nanocomposite polymer hydrogels. Colloid Polym. Sci. 2009, 287, 1–11. [Google Scholar] [CrossRef]
- Skardal, A.; Zhang, J.; McCoard, L.; Oottamasathien, S.; Prestwich, G.D. Dynamically crosslinked gold nanoparticle—Hyaluronan hydrogels. Adv. Mater. 2010, 22, 4736–4740. [Google Scholar] [CrossRef] [PubMed]
- Balazs, A.C.; Emrick, T.; Russell, T.P. Nanoparticle polymer composites: Where two small worlds meet. Science 2006, 314, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Vimala, K.; Sivudu, K.S.; Mohan, Y.M.; Sreedhar, B.; Raju, K.M. Controlled silver nanoparticles synthesis in semi-hydrogel networks of poly(acrylamide) and carbohydrates: A rational methodology for antibacterial application. Carbohydr. Polym. 2009, 75, 463–471. [Google Scholar] [CrossRef]
- Thomas, V.; Yallapu, M.M.; Sreedhar, B.; Bajpai, S.K. A versatile strategy to fabricate hydrogel-silver nanocomposites and investigation of their antimicrobial activity. J. Colloid Interface Sci. 2007, 315, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Ikeda, R.; Yanagida, Y.; Hatsuzawa, T. Stimuli-responsive hydrogel-silver nanoparticles composite for development of localized surface plasmon resonance-based optical biosensor. Anal. Chim. Acta 2008, 611, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Juby, K.A.; Dwivedi, C.; Kumar, M.; Kota, S.; Misra, H.S.; Bajaj, P.N. Silver nanoparticle-loaded PVA/gum acacia hydrogel: Synthesis, characterization and antibacterial study. Carbohydr. Polym. 2012, 89, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wu, J.; Kang, D.; Zhang, H. Development of a complex hydrogel of hyaluronan and PVA embedded with silver nanoparticles and its facile studies on Escherichia coli. J. Biomater. Sci. Polym. Ed. 2013, 24, 1410–1425. [Google Scholar] [CrossRef] [PubMed]
- Mohan, Y.M.; Premkumar, T.; Lee, K.; Geckeler, K.E. Fabrication of silver nanoparticles in hydrogel networks. Macromol. Rapid Commun. 2006, 27, 1346–1354. [Google Scholar] [CrossRef]
- Mbhele, Z.H.; Salemane, M.G.; van Sittert, C.G.C.E.; Nedeljkovic, J.M.; Djokovic, V.; Luyt, A.S. Fabrication and characterization of silver-polyvinyl alcohol nanocomposites. Chem. Mater. 2003, 15, 5019–5024. [Google Scholar] [CrossRef]
- Yadollahi, M.; Namazi, H.; Aghazadeh, M. Antibacterial carboxymethyl cellulose/Ag nanocomposite hydrogels cross-linked with layered double hydroxides. Int. J. Biol. Macromol. 2015, 79, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Chen, D. Preparation of a novel pH-responsive silver nanoparticle/poly (HEMA-PEGMA-MAA) composite hydrogel. Eur. Polym. J. 2007, 43, 4178–4187. [Google Scholar] [CrossRef]
- Boonkaew, B.; Barber, P.M.; Rengpipat, S.; Supaphol, P.; Kempf, M.; He, J.; John, V.T.; Cuttle, L. Development and characterization of a novel, antimicrobial, sterile hydrogel dressing for burn wounds: Single-step production with gamma irradiation creates silver nanoparticles and radical polymerization. J. Pharm. Sci. 2014, 103, 3244–3253. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Liu, B.; Wang, J.; Zhang, S.; Lin, Q.; Gong, P.; Ma, L.; Yang, S. A novel wound dressing based on Ag/graphene polymer hydrogel: Effectively kill bacteria and accelerate wound healing. Adv. Func. Mater. 2014, 24, 3933–3943. [Google Scholar] [CrossRef]
- Ahearn, D.G.; Grace, D.T.; Jennings, M.J.; Borazjani, R.N.; Boles, K.J.; Rose, L.J.; Simmons, R.B.; Ahanotu, E.N. Effects of hydrogel/silver coatings on in vitro adhesion to catheters of bacteria associated with urinary tract infections. Cur. Microbiol. 2000, 41, 120–125. [Google Scholar] [CrossRef]
- Rao, K.M.; Rao, K.S.; Ramanjaneyulu, G.; Rao, K.C.; Subha, M.C.; Ha, C.S. Biodegradable sodium alginate-based semi-interpenetrating polymer network hydrogels for antibacterial application. J. Biomed. Mater. Res. A 2014, 102, 3196–3206. [Google Scholar] [CrossRef] [PubMed]
- Reithofer, M.R.; Lakshmanan, A.; Ping, A.T.; Chin, J.M.; Hauser, C.A. In situ synthesis of size-controlled, stable silver nanoparticles within ultrashort peptide hydrogels and their anti-bacterial properties. Biomaterials 2014, 35, 7535–7542. [Google Scholar] [CrossRef] [PubMed]
- Manjula, B.; Varaprasad, K.; Sadiku, R.; Ramam, K.; Reddy, G.V.; Raju, K.M. Development of microbial resistant thermosensitive Ag nanocomposite (gelatin) hydrogels via green process. J. Biomed. Mater. Res. A 2014, 102, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Halim, E.S.; Al-Deyab, S.S. Electrically conducting silver/guar gum/poly(acrylic acid) nanocomposite. Int. J. Biol. Macromol. 2014, 69, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Chu, B.Y.; Peng, J.R.; Liao, J.F.; Qi, T.T.; Shi, K.; Zhang, X.N.; Wei, Y.Q.; Qian, Z.Y. A biodegradable thermo-responsive hybrid hydrogel: Therapeutic applications in preventing the post-operative recurrence of breast cancer. NPG Asia Mater. 2015, 7, e207. [Google Scholar] [CrossRef]
- Yu, J.; Ha, W.; Sun, J.; Shi, Y. Supramolecular Hybrid Hydrogel Based on Host-Guest Interaction and Its Application in Drug Delivery. ACS Appl. Mater. Interfaces 2014, 6, 19544–19551. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Chen, Q.; Huo, D.; Ding, Y.; Hu, Y.; Jiang, X. In situ formation of chitosan-gold hybrid hydrogel and its application for drug delivery. Colloid. Surface. B 2012, 97, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Hwang, S.; Im, K.; Hur, J.; Nam, J.; Hwang, S.; Ahn, G.O.; Kim, S.; Park, N. Light-responsible DNA hydrogel-gold nanoparticle assembly for synergistic cancer therapy. J. Mater. Chem. B 2015, 3, 1537–1543. [Google Scholar] [CrossRef]
- Wu, X.; Gao, Y.; Dong, C.M. Polymer/gold hybrid nanoparticles: From synthesis to cancer theranostic applications. RSC Adv. 2015, 5, 13787–13796. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, B.; Zhu, L.; Li, Y.; Huang, F.; Li, S.; Shen, Y.; Xie, A. Preparation and multiple antitumor properties of AuNRs/Spinach Extract/PEGDA composite hydrogel. ACS Appl. Mater. Interfaces 2014, 6, 15000–15006. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.; Park, J.Y.; Tung, C.H.; Kim, I.H.; Choi, Y. Gold nanorod-photosensitizer complex for near-infrared fluorescence imaging and photodynamic/photothermal therapy in vivo. ACS Nano 2011, 5, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Sershen, S.R.; Westcott, S.L.; Halas, N.J.; West, J.L. Temperature-sensitive polymer-nanoshell composites for photothermally modulated drug delivery. J. Biomed. Mater. Res. 2000, 5, 293–298. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Wagner, B.; Karbarz, M.; Nowicka, A.M. A dual DNA biosensor based on two redox couples with a hydrogel sensing platform functionalized with carboxyl groups and gold nanoparticles. Sensor. Actuat. B 2015, 208, 220–227. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Yan, C.; Liu, P.; Ding, J. Fabrication of RGD micro/nanopattern and corresponding study of stem cell differentiation. Nano Lett. 2015, 15, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.N.; Ko, W.K.; Bae, M.S.; Lee, J.B.; Lee, D.W.; Byun, W.; Lee, C.H.; Kim, E.C.; Jung, B.-Y.; Kwon, I.K. Enhanced bone regeneration with a gold nanoparticle-hydrogel complex. J. Mater. Chem. B 2014, 2, 1584–1593. [Google Scholar] [CrossRef]
- Pérez, R.A.; Won, J.E.; Knowles, J.C.; Kim, H.W. Naturally and synthetic smart composite biomaterials for tissue regeneration. Adv. Drug Deliv. Rev. 2013, 65, 471–496. [Google Scholar] [CrossRef] [PubMed]
- Samal, S.K.; Dash, M.; Shelyakova, T.; Declercq, H.A.; Uhlarz, M.; Banobre-Lopez, M.; Dubruel, P.; Cornelissen, M.; Herrmannsdorfer, T.; Rivas, J.; et al. Biomimetic magnetic silk scaffolds. ACS Appl. Mater. Interfaces 2015, 7, 6282–6292. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Hu, S.H.; Liu, T.Y.; Liu, D.M.; Chen, S.Y. Magnetic-sensitive behavior of intelligent ferrogels for controlled release of drug. Langmuir 2006, 22, 5974–5978. [Google Scholar] [CrossRef] [PubMed]
- Satarkar, N.S.; Zach Hilt, J. Hydrogel nanocomposites as remote-controlled biomaterials. Acta Biomater. 2008, 4, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Ghaemy, M.; Naseri, M. Synthesis of chitosan networks: Swelling, drug release, and magnetically assisted BSA separation using Fe3O4 nanoparticles. Carbohydr. Polym. 2012, 90, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, L.; Cheng, R.; Mao, L.; Arnold, R.D.; Howerth, E.W.; Chen, Z.G.; Platt, S. Magnetic nanoparticle-based hyperthermia for head & neck cancer in mouse models. Theranostics 2012, 2, 113–121. [Google Scholar] [PubMed]
- Lee, N.; Cho, H.R.; Oh, M.H.; Lee, S.H.; Kim, K.; Kim, B.H.; Shin, K.; Ahn, T.-Y.; Choi, J.W.; Kim, Y.-W. Multifunctional Fe3O4/TaOx Core/Shell Nanoparticles for Simultaneous Magnetic Resonance Imaging and X-ray Computed Tomography. J. Am. Chem. Soc. 2012, 134, 10309–10312. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Lock, J.; Sallee, A.; Liu, H. Magnetic Nanocomposite Hydrogel for Potential Cartilage Tissue Engineering: Synthesis, Characterization, and Cytocompatibility with Bone Marrow Derived Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2015, 7, 20987–20998. [Google Scholar] [CrossRef] [PubMed]
- Thiruvengadam, V.; Vitta, S. Ni–bacterial cellulose nanocomposite: A magnetically active inorganic–organic hybrid gel. RSC Adv. 2013, 3, 12765–12773. [Google Scholar] [CrossRef]
- Fuhrer, R.; Athanassiou, E.K.; Luechinger, N.A.; Stark, W.J. Crosslinking metal nanoparticles into the polymer backbone of hydrogels enables preparation of soft, magnetic field-driven actuators with muscle-like flexibility. Small 2009, 5, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Price, R.L.; Gutwein, L.G.; Kaledin, L.; Tepper, F.; Webster, T.J. Osteoblast function on nanophase alumina materials: Influence ofchemistry, phase, and topography. J. Biomed. Mater. Res. A 2003, 67, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Siegel, R.W.; Bizios, R. Nanoceramic surface roughness enhances osteoblast and osteoclast functions for improved orthopaedic/dental implant efficacy. Scr. Mater. 2001, 44, 1639–1642. [Google Scholar] [CrossRef]
- Vimala, K.; Kanny, K.; Varaprasad, K.; Kumar, N.M.; Reddy, G.S. Novel-porous-Ag0 nanocomposite hydrogels via green process for advanced antibacterial applications. J. Biomed. Mater. Res. A 2014, 102, 4616–4624. [Google Scholar] [PubMed]
- Vimala, K.; Varaprasad, K.; Sadiku, R.; Ramam, K.; Kanny, K. Development of novel protein-Ag nanocomposite for drug delivery and inactivation of bacterial applications. Int. J. Biol. Macromol. 2014, 63, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, K.; Mohan, Y.M.; Vimala, K.; Raju, K.M. Synthesis and characterization of hydrogel-silver nanoparticle-curcumin composites for wound dressing and antibacterial application. J. Appl. Polym. Sci. 2011, 121, 784–796. [Google Scholar] [CrossRef]
- Murthy, P.S.K.; Mohan, Y.M.; Varaprasad, K.; Sreedhar, B.; Raju, K.M. First successful design of semi-IPN hydrogel-silver nanocomposites: A facile approach for antibacterial application. J. Colloid Interface Sci. 2008, 318, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Varaprasad, K.; Mohan, Y.M.; Ravindra, S.; Reddy, N.N.; Vimala, K.; Monika, K.; Sreedhar, B.; Raju, K.M. Hydrogel-silver nanoparticle composites: A new generation of antimicrobials. J. Appl. Polym. Sci. 2010, 115, 1199–1207. [Google Scholar] [CrossRef]
- Jovanović, Ž.; Radosavljević, A.; Stojkovska, J.; Nikolić, B.; Obradovic, B.; Kačarević-Popović, Z.; Mišković-Stanković, V. Silver/poly(N-vinyl-2-pyrrolidone) hydrogel nanocomposites obtained by electrochemical synthesis of silver nanoparticles inside the polymer hydrogel aimed for biomedical applications. Polym. Compos. 2014, 35, 217–226. [Google Scholar] [CrossRef]
- Oliveira, R.N.; Rouze, R.; Quilty, B.; Alves, G.G.; Soares, G.D.A.; Thire, R.M.; McGuinness, G.B. Mechanical properties and in vitro characterization of polyvinyl alcohol-nano-silver hydrogel wound dressings. Interface Focus 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, H.; Ghanaat, F. Antimicrobial alginate/PVA silver nanocomposite hydrogel, synthesis and characterization. J. Polym. Res. 2014, 21, 1–14. [Google Scholar] [CrossRef]
- Eghbalifam, N.; Frounchi, M.; Dadbin, S. Antibacterial silver nanoparticles in polyvinyl alcohol/sodium alginate blend produced by gamma irradiation. Int. J. Biol. Macromol. 2015, 80, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Zan, X.; Kozlov, M.; McCarthy, T.J.; Su, Z. Covalently attached, silver-doped poly(vinyl alcohol) hydrogel films on poly(l-lactic acid). Biomacromolecules 2010, 11, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Deen, G.; Chua, V. Synthesis and properties of new “stimuli” responsive nanocomposite hydrogels containing silver nanoparticles. Gels 2015, 1, 117–134. [Google Scholar] [CrossRef]
- Spasojević, J.; Radosavljević, A.; Krstić, J.; Jovanović, D.; Spasojević, V.; Kalagasidis-Krušić, M.; Kačarević-Popović, Z. Dual responsive antibacterial Ag-poly(N-isopropylacrylamide/itaconic acid) hydrogel nanocomposites synthesized by gamma irradiation. Eur. Polym. J. 2015, 69, 168–185. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Bajpai, M.; Sharma, L. In situ formation of silver nanoparticles in poly(N-isopropyl acrylamide) hydrogel for antibacterial applications. Des. Monomers Polym. 2011, 14, 383–394. [Google Scholar] [CrossRef]
- Bazzaz, B.S.F.; Khameneh, B.; Jalili-Behabadi, M.M.; Malaekeh-Nikouei, B.; Mohajeri, S.A. Preparation, characterization and antimicrobial study of a hydrogel (soft contact lens) material impregnated with silver nanoparticles. Cont. Lens. Anterio Eye 2014, 37, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Guadalupe Rodriguez-Delgado, M.; Yanez-Flores, I.G.; Sanchez-Valdes, S.; Rodriguez-Fernandez, O.S.; Betancourt-Galindo, R.; Lozano-Ramirez, T.; Ramirez-Vargas, E.; Ortega-Ortiz, H. Preparation and characterization of acrylic acid/itaconic acid hydrogel coatings containing silver nanoparticles. J. Appl. Polym. Sci. 2013, 130, 2713–2721. [Google Scholar] [CrossRef]
- Wei, Q.B.; Fu, F.; Zhang, Y.Q.; Tang, L. Preparation, characterization, and antibacterial properties of pH-responsive P(MMA-co-MAA)/silver nanocomposite hydrogels. J. Polym. Res. 2014, 21, 1–9. [Google Scholar] [CrossRef]
- Gils, P.S.; Ray, D.; Sahoo, P.K. Designing of silver nanoparticles in gum arabic based semi-IPN hydrogel. Int. J. Biol. Mmacromol. 2010, 46, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-A.; Sung, A.Y. Change of physical properties of hydrogel lens polymer containing isocyanate group with Ag nanoparticle. J. Chosun Nat. Sci. 2014, 7, 5–10. [Google Scholar] [CrossRef]
- Boonkaew, B.; Kempf, M.; Kimble, R.; Supaphol, P.; Cuttle, L. Antimicrobial efficacy of a novel silver hydrogel dressing compared to two common silver burn wound dressings: Acticoat™ and PolyMem Silver®. Burns 2014, 40, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Young Yook, J.; Choi, G.H.; Hack Suh, D. A novel method for preparing silver nanoparticle-hydrogel nanocomposites via pH-induced self-assembly. Chem. Commun. 2012, 48, 5001–5003. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.K.; Brahmachari, S.; Das, P.K. In situ synthesised silver nanoparticle-infusedl-lysine-based injectable hydrogel: Development of a biocompatible, antibacterial, soft nanocomposite. ChemPlusChem 2014, 79, 1733–1746. [Google Scholar] [CrossRef]
- Adhikari, B.; Banerjee, A. Short-peptide-based hydrogel: A template for the in situ synthesis of fluorescent silver nanoclusters by using sunlight. Chem. Eur. J. 2010, 16, 13698–13705. [Google Scholar] [CrossRef] [PubMed]
- Bardajee, G.R.; Hooshyar, Z.; Rezanezhad, H. A novel and green biomaterial based silver nanocomposite hydrogel: Synthesis, characterization and antibacterial effect. J. Inorg. Biochem. 2012, 117, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Kumar, A.; Patil, N.B.; Viswanathan, C.; Ghosh, D. Preparation and characterization of silver nanoparticle loaded amorphous hydrogel of carboxymethylcellulose for infected wounds. Carbohydr. Polym. 2015, 130, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Hebeish, A.; Hashem, M.; El-Hady, M.M.A.; Sharaf, S. Development of CMC hydrogels loaded with silver nano-particles for medical applications. Carbohydr. Polym. 2013, 92, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Rattanaruengsrikul, V.; Pimpha, N.; Supaphol, P. In vitro efficacy and toxicology evaluation of silver nanoparticle-loaded gelatin hydrogel pads as antibacterial wound dressings. J. Appl. Polym. Sci. 2012, 124, 1668–1682. [Google Scholar] [CrossRef]
- Sahiner, M.; Alpaslan, D.; Bitlisli, B.O. Collagen-based hydrogel films as drug-delivery devices with antimicrobial properties. Polym. Bull. 2014, 71, 3017–3033. [Google Scholar] [CrossRef]
- Garcia-Astrain, C.; Chen, C.; Buron, M.; Palomares, T.; Eceiza, A.; Fruk, L.; Corcuera, M.A.; Gabilondo, N. Biocompatible hydrogel nanocomposite with covalently embedded silver nanoparticles. Biomacromolecules 2015, 16, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, T.J.; Rao, K.S.; Kumar, A. Facile preparation of agarose–chitosan hybrid materials and nanocomposite ionogels using an ionic liquid via dissolution, regeneration and sol–gel transition. Green Chem. 2014, 16, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Martins, A.F.; Monteiro, J.P.; Bonafe, E.G.; Gerola, A.P.; Silva, C.T.P.; Girotto, E.M.; Rubira, A.F.; Muniz, E.C. Bactericidal activity of hydrogel beads based on N,N,N-trimethyl chitosan/alginate complexes loaded with silver nanoparticles. Chin. Chem. Lett. 2015, 26, 1129–1132. [Google Scholar] [CrossRef]
- Grade, S.; Eberhard, J.; Neumeister, A.; Wagener, P.; Winkel, A.; Stiesch, M.; Barcikowski, S. Serum albumin reduces the antibacterial and cytotoxic effects of hydrogel-embedded colloidal silver nanoparticles. RSC Adv. 2012, 2, 7190–7196. [Google Scholar] [CrossRef]
- Hauser, A.W.; Evans, A.A.; Na, J.-H.; Hayward, R.C. Photothermally reprogrammable buckling of nanocomposite gel sheets. Angew. Chem. Int. Ed. 2015, 54, 5434–5437. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, T.R. Hydrogel-templated growth of large gold nanoparticles: Synthesis of thermally responsive hydrogel-nanoparticle composites. Langmuir 2007, 23, 6504–6509. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, T.R. Discrete thermally responsive hydrogel-coated gold nanoparticles for use as drug-delivery vehicles. Drug Dev. Res. 2006, 67, 61–69. [Google Scholar] [CrossRef]
- Rong, Q.; Han, H.; Feng, F.; Ma, Z. Network nanostructured polypyrrole hydrogel/Au composites as enhanced electrochemical biosensing platform. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, D.; Sasaki, M.; Nagamine, K.; Torimitsu, K.; Nishizawa, M. Electroporation of adherent cells by direct lamination of hydrogel-based microelectrode substrates. Chem. Lett. 2014, 43, 444–446. [Google Scholar] [CrossRef]
- Cheng, C.; Tang, M.C.; Wu, C.S.; Simon, T.; Ko, F.-H. New synthesis route of hydrogel through a bioinspired supramolecular approach: Gelation, binding interaction, and in vitro dressing. ACS Appl. Mater. Interfaces 2015, 7, 19306–19315. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ma, Y.; Chen, Y.; Wu, X.; Fang, L.; Zhu, Z.; Yang, C.J. Target-responsive DNAzyme cross-linked hydrogel for visual quantitative detection of lead. Anal. Chem. 2014, 86, 11434–11439. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, J.; Gao, L.; Li, C.M. Au nanoparticles-3D graphene hydrogel nanocomposite to boost synergistically in situ detection sensitivity toward cell-released nitric oxide. ACS Appl. Mater. Interfaces 2015, 7, 2726–2734. [Google Scholar] [CrossRef] [PubMed]
- Mahdavinia, G.R.; Etemadi, H.; Soleymani, F. Magnetic/pH-responsive beads based on caboxymethyl chitosan and kappa-carrageenan and controlled drug release. Carbohydr. Polym. 2015, 128, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Mahdavinia, G.R.; Etemadi, H. In situ synthesis of magnetic CaraPVA IPN nanocomposite hydrogels and controlled drug release. Mater. Sci. Eng. C 2014, 45, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Bardajee, G.R.; Hooshyar, Z.; Rastgo, F. Kappa carrageenan-g-poly (acrylic acid)/SPION nanocomposite as a novel stimuli-sensitive drug delivery system. Colloid Polym. Sci. 2013, 291, 2791–2803. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Rahmani, Z.; Karami, S.; Pourjavadi, A. Magnetic/pH-sensitive kappa-carrageenan/sodium alginate hydrogel nanocomposite beads: Preparation, swelling behavior, and drug delivery. J. Biomater. Sci. Polym. Ed. 2014, 25, 1891–1906. [Google Scholar] [CrossRef] [PubMed]
- Bardajee, G.R.; Hooshyar, Z. One-pot synthesis of biocompatible superparamagnetic iron oxide nanoparticles/hydrogel based on salep: Characterization and drug delivery. Carbohydr. Polym. 2014, 101, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Bardajee, G.R.; Hooshyar, Z.; Asli, M.J.; Shahidi, F.E.; Dianatnejad, N. Synthesis of a novel supermagnetic iron oxide nanocomposite hydrogel based on graft copolymerization of poly((2-dimethylamino)ethyl methacrylate) onto salep for controlled release of drug. Mater. Sci. Eng. C 2014, 36, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Bardajee, G.R.; Hooshyar, Z. A novel biocompatible magnetic iron oxide nanoparticles/hydrogel based on poly (acrylic acid) grafted onto starch for controlled drug release. J. Polym. Res. 2013, 20, 1–13. [Google Scholar] [CrossRef]
- Uva, M.; Mencuccini, L.; Atrei, A.; Innocenti, C.; Fantechi, E.; Sangregorio, C.; Maglio, M.; Fini, M.; Barbucci, R. On the Mechanism of drug release from polysaccharide hydrogels cross-linked with magnetite nanoparticles by applying alternating magnetic fields: The case of Dox delivery. Gels 2015, 1, 24–43. [Google Scholar] [CrossRef]
- Giani, G.; Fedi, S.; Barbucci, R. Hybrid magnetic hydrogel: A potential system for controlled drug delivery by means of alternating magnetic fields. Polymers 2012, 4, 1157–1169. [Google Scholar] [CrossRef]
- Ziv-Polat, O.; Skaat, H.; Shahar, A.; Margel, S. Novel magnetic fibrin hydrogel scaffolds containing thrombin and growth factors conjugated iron oxide nanoparticles for tissue engineering. Int. J. Nanomed. 2012, 7, 1259–1274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, Y.; Yang, X.; Hilborn, J.; Heerschap, A.; Ossipov, D.A. Injectable in situ forming hybrid iron oxide-hyaluronic acid hydrogel for magnetic resonance imaging and drug delivery. Macromol. Biosci. 2014, 14, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Sun, P.; Li, P.; Xue, A.; Zhang, X.; Zhang, H.; Jin, X. A magnetic chitosan hydrogel for sustained and prolonged delivery of Bacillus Calmette-Guerin in the treatment of bladder cancer. Biomaterials 2013, 34, 10258–10266. [Google Scholar] [CrossRef] [PubMed]
- Demarchi, C.A.; Debrassi, A.; Buzzi Fde, C.; Correa, R.; Filho, V.C.; Rodrigues, C.A.; Nedelko, N.; Demchenko, P.; Slawska-Waniewska, A.; Dluzewski, P.; et al. A magnetic nanogel based on O-carboxymethylchitosan for antitumor drug delivery: Synthesis, characterization and in vitro drug release. Soft Matter 2014, 10, 3441–3450. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Li, P.; Li, Y.M.; Wang, A.Q. Preparation and characterization of magnetic alginate-chitosan hydrogel beads loaded matrine. Drug Dev. Ind. Pharm. 2012, 38, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.B.; Pan, L.; Zhang, K.; Guo, S.S.; Liu, W.; Wang, Y.; Chen, Y.; Zhao, X.Z.; Chan, H.L.W. Generation of Janus alginate hydrogel particles with magnetic anisotropy for cell encapsulation. Lab Chip 2009, 9, 2981–2986. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Vidhya, P.; Thanikaivelan, P. Thermoresponsive magnetic nanoparticle-aminated guar gum hydrogel system for sustained release of doxorubicin hydrochloride. Carbohydr. Polym. 2014, 110, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.I.; Lee, B.S.; Chun, C.; Cho, J.-K.; Kim, S.-Y.; Song, S.-C. Long-term theranostic hydrogel system for solid tumors. Biomaterials 2012, 33, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Koc, K.; Alveroglu, E. Adsorption and desorption studies of lysozyme by Fe3O4-polymer nanocomposite via fluorescence spectroscopy. J. Mol. Struc. 2015, 1089, 66–72. [Google Scholar] [CrossRef]
- Reddy, N.N.; Mohan, Y.M.; Varaprasad, K.; Ravindra, S.; Joy, P.A.; Raju, K.M. Magnetic and electric responsive hydrogel-magnetic nanocomposites for drug-delivery application. J. Appl. Polym. Sci. 2011, 122, 1364–1375. [Google Scholar] [CrossRef]
- Reddy, N.N.; Varaprasad, K.; Ravindra, S.; Reddy, G.V.S.; Reddy, K.M.S.; Reddy, K.M.M.; Raju, K.M. Evaluation of blood compatibility and drug release studies of gelatin based magnetic hydrogel nanocomposites. Colloid. Surface. A 2011, 385, 20–27. [Google Scholar] [CrossRef]
- Hu, K.; Sun, J.; Guo, Z.; Wang, P.; Chen, Q.; Ma, M.; Gu, N. A novel magnetic hydrogel with aligned magnetic colloidal assemblies showing controllable enhancement of magnetothermal effect in the presence of alternating magnetic field. Adv. Mater. 2015, 27, 2507–2514. [Google Scholar] [CrossRef] [PubMed]
- Satarkar, N.S.; Hilt, J.Z. Magnetic hydrogel nanocomposites for remote controlled pulsatile drug release. J. Control. Release 2008, 130, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Nazli, C.; Demirer, G.S.; Yar, Y.; Acar, H.Y.; Kizilel, S. Targeted delivery of doxorubicin into tumor cells via MMP-sensitive PEG hydrogel-coated magnetic iron oxide nanoparticles (MIONPs). Colloid. Surface. B 2014, 122, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Nazli, C.; Ergenc, T.I.; Yar, Y.; Acar, H.Y.; Kizilel, S. RGDS-functionalized polyethylene glycol hydrogel-coated magnetic iron oxide nanoparticles enhance specific intracellular uptake by HeLa cells. Int. J. Nanomed. 2012, 7, 1903–1920. [Google Scholar]
- Adriane, K.; Huang, J.; Ding, G.W.; Chen, J.; Liu, Y.F. Self assembled magnetic PVP/PVA hydrogel microspheres; magnetic drug targeting of VX2 auricular tumours using pingyangmycin. J. Drug Target. 2006, 14, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.Y.; Lin, C.Y.; Chu, W.C.; Chang, H.Y. A simple cell patterning method using magnetic particle-containing photosensitive poly (ethylene glycol) hydrogel blocks: A Technical Note. Tissue Eng. C 2011, 17, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Mahdavinia, G.R.; Ettehadi, S.; Amini, M.; Sabzi, M. Synthesis and characterization of hydroxypropyl methylcellulose-g-poly (acrylamide)/LAPONITE® nanocomposites as novel magnetic-and pH-sensitive carriers for controlled drug release. RSC Adv. 2015, 5, 44516–44523. [Google Scholar] [CrossRef]
- Chou, F.Y.; Shih, C.M.; Tsai, M.C.; Chiu, W.Y.; Lue, S.J. Functional acrylic acid as stabilizer for synthesis of smart hydrogel particles containing a magnetic Fe3O4 core. Polymer 2012, 53, 2839–2846. [Google Scholar] [CrossRef]
- Fan, T.; Li, M.; Wu, X.; Li, M.; Wu, Y. Preparation of thermoresponsive and pH-sensitivity polymer magnetic hydrogel nanospheres as anticancer drug carriers. Colloid. Surface. B 2011, 88, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Divya, P.L.; Nima, J. Synthesis and characterization of silane coated magnetic nanoparticles/glycidylmethacrylate-grafted-maleated cyclodextrin composite hydrogel as a drug carrier for the controlled delivery of 5-fluorouracil. Mater. Sci. Eng. C 2015, 55, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Bayramoglu, G.; Altintas, B.; Arica, M.Y. Immobilization of glucoamylase onto polyaniline-grafted magnetic hydrogel via adsorption and adsorption/cross-linking. Appl. Microbiol. Biotechnol. 2013, 97, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, R.; Goswami, U.; Ghosh, S.S.; Paul, A.; Chattopadhyay, A. Synergistic anticancer activity of fluorescent copper nanoclusters and cisplatin delivered through a hydrogel nanocarrier. ACS Appl. Mater. Interfaces 2015, 7, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Cometa, S.; Iatta, R.; Ricci, M.A.; Ferretti, C.; de Giglio, E. Analytical characterization and antimicrobial properties of novel copper nanoparticle-loaded electrosynthesized hydrogel coatings. J. Bioact. Compat. Polym. 2013, 28, 508–522. [Google Scholar] [CrossRef]
- Yadollahi, M.; Gholamali, I.; Namazi, H.; Aghazadeh, M. Synthesis and characterization of antibacterial carboxymethylcellulose/CuO bio-nanocomposite hydrogels. Int. J. Biol. Macromol. 2015, 73, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Staneva, D.; Atanasova, D.; Vasileva-Tonkova, E.; Lukanova, V.; Grabchev, I. A cotton fabric modified with a hydrogel containing ZnO nanoparticles: Preparation and properties study. Appl. Surf. Sci. 2015, 345, 72–80. [Google Scholar] [CrossRef]
- Yadollahi, M.; Gholamali, I.; Namazi, H.; Aghazadeh, M. Synthesis and characterization of antibacterial carboxymethyl cellulose/ZnO nanocomposite hydrogels. Int. J. Biol. Macromol. 2015, 74, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Hashem, M.; Sharaf, S.; Abd El-Hady, M.M.; Hebeish, A. Synthesis and characterization of novel carboxymethylcellulose hydrogels and carboxymethylcellulolse hydrogel ZnO nanocomposites. Carbohydr. Polym. 2013, 95, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.P.T.; Lakshmanan, V.K.; Raj, M.; Biswas, R.; Hiroshi, T.; Nair, S.V.; Jayakumar, R. Evaluation of wound healing potential of beta-chitin hydrogel/nano Zinc oxide composite bandage. Pharm. Res. 2013, 30, 523–537. [Google Scholar]
- Zhang, Q.; Fang, Z.; Cao, Y.; Du, H.; Wu, H.; Beuerman, R.; Chan-Park, M.B.; Duan, H.; Xu, R. High refractive index inorganic–organic interpenetrating polymer network (IPN) hydrogel nanocomposite toward artificial cornea implants. ACS Macro Lett. 2012, 1, 876–881. [Google Scholar] [CrossRef]
- Weltin, A.; Kieninger, J.; Enderle, B.; Gellner, A.K.; Fritsch, B.; Urban, G.A. Polymer-based, flexible glutamate and lactate microsensors for in vivo applications. Biosens. Bioelectron. 2014, 61, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.H.; Zhang, H.; Xu, J.J.; Chen, H.Y. Relationship between nanostructure and electrochemical/biosensing properties of MnO2 nanomaterials for H2O2/choline. J. Phys. Chem. C 2008, 112, 18984–18990. [Google Scholar] [CrossRef]
- Xu, B.; Jiang, H.; Li, H.; Zhang, G.; Zhang, Q. High strength nanocomposite hydrogel bilayer with bidirectional bending and shape switching behaviors for soft actuators. RSC Adv. 2015, 5, 13167–13170. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Yao, D.; Yang, B. Hybridization of inorganic nanoparticles and polymers to create regular and reversible self-assembly architectures. Chem. Soc. Rev. 2012, 41, 6066–6088. [Google Scholar] [CrossRef] [PubMed]
- Kastellorizios, M.; Papadimitrakopoulos, F.; Burgess, D.J. Multiple tissue response modifiers to promote angiogenesis and prevent the foreign body reaction around subcutaneous implants. J. Control. Release 2015, 214, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Papadimitrakopoulos, F.; Burgess, D.J. Polymeric “smart” coatings to prevent foreign body response to implantable biosensors. J. Control. Release 2013, 169, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Kastellorizios, M.; Papadimitrakopoulos, F.; Burgess, D.J. Prevention of foreign body reaction in a pre-clinical large animal model. J. Control. Release 2015, 202, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Burgess, D.J. Prediction of dexamethasone release from PLGA microspheres prepared with polymer blends using a design of experiment approach. Int. J. Pharm. 2015, 495, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Zhang, G.H.; Hou, R.X.; Xu, H.P.; Li, Y.P.; Fu, J. Controllable promotion of chondrocyte adhesion and growth on PVA hydrogels by controlled release of TGF-beta 1 from porous PLGA microspheres. Colloid. Surface. B 2015, 125, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.H.; Hou, R.X.; Zhan, D.X.; Cong, Y.; Cheng, Y.J.; Fu, J. Fabrication of hollow porous PLGA microspheres for controlled protein release and promotion of cell compatibility. Chin. Chem. Lett. 2013, 24, 710–714. [Google Scholar] [CrossRef]
- Rahmani-Neishaboor, E.; Hartwell, R.; Jalili, R.; Jackson, J.; Brown, E.; Ghahary, A. Localized controlled release of stratifin reduces implantation-induced dermal fibrosis. Acta Biomater. 2012, 8, 3660–3668. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.H.; Choi, S.W.; Choi, J.W.; Paik, D.H.; Kang, S.S.; Kim, S.E.; Jeon, Y.-C.; Huh, J.B. Effects of different rhBMP-2 release profiles in defect areas around dental implants on bone regeneration. Biomed. Mater. 2015, 10, 045007. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.M.; Lewis, J.S.; Carstens, M.R.; Campbell-Thompson, M.; Wasserfall, C.H.; Atkinson, M.A.; Keselowsky, B.G. A combination hydrogel microparticle-based vaccine prevents type 1 diabetes in non-obese diabetic mice. Sci. Rep. 2015, 5, 13155. [Google Scholar] [CrossRef] [PubMed]
- Sellers, D.L.; Kim, T.H.; Mount, C.W.; Pun, S.H.; Horner, P.J. Poly(lactic-co-glycolic) acid microspheres encapsulated in Pluronic F-127 prolong hirudin delivery and improve functional recovery from a demyelination lesion. Biomaterials 2014, 35, 8895–8902. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.Q.; Song, H.; Qi, J.; Cui, D.X. Sustained release of VEGF from PLGA nanoparticles embedded thermo-sensitive hydrogel in full-thickness porcine bladder acellular matrix. Nanoscale Res. Lett. 2011, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Karam, J.P.; Muscari, C.; Sindji, L.; Bastiat, G.; Bonafè, F.; Venier-Julienne, M.-C.; Montero-Menei, N.C. Pharmacologically active microcarriers associated with thermosensitive hydrogel as a growth factor releasing biomimetic 3D scaffold for cardiac tissue-engineering. J. Control. Release 2014, 192, 82–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acarregui, A.; Herrán, E.; Igartua, M.; Blanco, F.J.; Pedraz, J.L.; Orive, G.; Hernandez, R.M. Multifunctional hydrogel-based scaffold for improving the functionality of encapsulated therapeutic cells and reducing inflammatory response. Acta Biomater. 2014, 10, 4206–4216. [Google Scholar] [CrossRef] [PubMed]
- Yonet-Tanyeri, N.; Rich, M.H.; Lee, M.; Lai, M.H.; Jeong, J.H.; DeVolder, R.J.; Kong, H. The spatiotemporal control of erosion and molecular release from micropatterned poly(ethylene glycol)-based hydrogel. Biomaterials 2013, 34, 8416–8423. [Google Scholar] [CrossRef] [PubMed]
- Elliott Donaghue, I.; Tator, C.H.; Shoichet, M.S. Sustained delivery of bioactive neurotrophin-3 to the injured spinal cord. Biomater. Sci. 2015, 3, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Elliott Donaghue, I.; Shoichet, M.S. Controlled release of bioactive PDGF-AA from a hydrogel/nanoparticle composite. Acta Biomater. 2015, 25, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; Cooke, M.J.; Sachewsky, N.; Morshead, C.M.; Shoichet, M.S. Bioengineered sequential growth factor delivery stimulates brain tissue regeneration after stroke. J. Control. Release 2013, 172, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.E.; Baumann, M.D.; Tator, C.H.; Shoichet, M.S. Localized and sustained delivery of fibroblast growth factor-2 from a nanoparticle-hydrogel composite for treatment of spinal cord injury. Cells Tissues Organs 2013, 197, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Stanwick, J.C.; Baumann, M.D.; Shoichet, M.S. Enhanced neurotrophin-3 bioactivity and release from a nanoparticle-loaded composite hydrogel. J. Control. Release 2012, 160, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Stanwick, J.C.; Baumann, M.D.; Shoichet, M.S. In vitro sustained release of bioactive anti-NogoA, a molecule in clinical development for treatment of spinal cord injury. Int. J. Pharm. 2012, 426, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.D.; Kang, C.E.; Tator, C.H.; Shoichet, M.S. Intrathecal delivery of a polymeric nanocomposite hydrogel after spinal cord injury. Biomaterials 2010, 31, 7631–7639. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gao, W.W.; Thamphiwatana, S.; Luk, B.T.; Angsantikul, P.; Zhang, Q.Z.; Hu, C.M.J.; Fang, R.H.; Copp, J.A.; Pornpattananangkul, D.; et al. Hydrogel retaining toxin-absorbing nanosponges for local treatment of methicillin-resistant staphylococcus aureus infection. Adv. Mater. 2015, 27, 3437–3443. [Google Scholar] [CrossRef] [PubMed]
- Pakulska, M.M.; Vulic, K.; Tam, R.Y.; Shoichet, M.S. Hybrid crosslinked methylcellulose hydrogel: A predictable and tunable platform for local drug delivery. Adv. Mater. 2015, 27, 5002–5008. [Google Scholar] [CrossRef] [PubMed]
- Posadowska, U.; Parizek, M.; Filova, E.; Wlodarczyk-Biegun, M.; Kamperman, M.; Bacakova, L.; Pamula, E. Injectable nanoparticle-loaded hydrogel system for local delivery of sodium alendronate. Int. J. Pharm. 2015, 485, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Baghaei, B.; Jafari, S.H.; Khonakdar, H.A.; Wagenknecht, U.; Heinrich, G. Novel thermosensitive hydrogel composites based on poly(d,l-lactide-co-glycolide) nanoparticles embedded in poly(N-isopropyl acrylamide) with sustained drug-release behavior. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Yang, H.; Tyagi, P.; Kadam, R.S.; Holden, C.A.; Kompella, U.B. Hybrid dendrimer hydrogel/PLGA nanoparticle platform sustains drug delivery for one week and antiglaucoma effects for four days following one-time topical administration. ACS Nano 2012, 6, 7595–7606. [Google Scholar] [CrossRef] [PubMed]
- Chronopoulou, L.; Sennato, S.; Bordi, F.; Giannella, D.; di Nitto, A.; Barbetta, A.; Dentini, M.; Togna, A.R.; Togna, G.I.; Moschini, S.; et al. Designing unconventional Fmoc-peptide-based biomaterials: Structure and related properties. Soft Matter 2014, 10, 1944–1952. [Google Scholar] [CrossRef] [PubMed]
- Gause, S.; Chauhan, A. Nanoparticle-loaded UV-blocking contact lenses. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Rossi, F.; Ferrari, R.; Castiglione, F.; Mele, A.; Perale, G.; Moscatelli, D. Polymer hydrogel functionalized with biodegradable nanoparticles as composite system for controlled drug delivery. Nanotechnology 2015, 26, 015602. [Google Scholar] [CrossRef] [PubMed]
- Murab, S.; Samal, J.; Shrivastava, A.; Ray, A.R.; Pandit, A.; Ghosh, S. Glucosamine loaded injectable silk-in-silk integrated system modulate mechanical properties in bovine ex vivo degenerated intervertebral disc model. Biomaterials 2015, 55, 64–83. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, D.P.; Amaral, M.H.; Reis, R.L.; Marques, A.P.; Correlo, V.M. Development of an injectable PHBV microparticles-GG hydrogel hybrid system for regenerative medicine. Int. J. Pharm. 2015, 478, 398–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appel, E.A.; Tibbitt, M.W.; Webber, M.J.; Mattix, B.A.; Veiseh, O.; Langer, R. Self-assembled hydrogels utilizing polymer-nanoparticle interactions. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Samanta, D.; Meiser, J.L.; Zare, R.N. Polypyrrole nanoparticles for tunable, pH-sensitive and sustained drug release. Nanoscale 2015, 7, 9497–9504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, R.C.; Lim, Z.H.; Li, W.; Shi, P.; Chen, C.H. Near-infrared light triggerable deformation-free polysaccharide double network hydrogels. Chem. Commun. 2014, 50, 7052–7055. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Sun, X.; Gong, T.; Wu, C.Y.; Zhang, T.; Tan, J.; Zhang, Z.R. Injectable and biodegradable thermosensitive hydrogels loaded with PHBHHx nanoparticles for the sustained and controlled release of insulin. Acta Biomater. 2013, 9, 5063–5069. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Abou-Jaoude, M.; Carbia, B.E.; Plummer, C.; Chauhan, A. Glaucoma therapy by extended release of timolol from nanoparticle loaded silicone-hydrogel contact lenses. J. Control. Release 2013, 165, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Dyondi, D.; Webster, T.J.; Banerjee, R. A nanoparticulate injectable hydrogel as a tissue engineering scaffold for multiple growth factor delivery for bone regeneration. Int. J. Nanomed. 2013, 8, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Ferrari, R.; Papa, S.; Moscatelli, D.; Casalini, T.; Forloni, G.; Perale, G.; Veglianese, P. Tunable hydrogel-Nanoparticles release system for sustained combination therapies in the spinal cord. Colloid. Surface. B 2013, 108, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Segovia, N.; Pont, M.; Oliva, N.; Ramos, V.; Borrós, S.; Artzi, N. Hydrogel doped with nanoparticles for local sustained release of siRNA in breast cancer. Adv. Healthc. Mater. 2015, 4, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Guo, Q.F.; Luo, J.C.; Luo, F.; Xie, P.; Tang, X.H.; Qian, Z.Y. Preparation and in vitro characterization of dexamethasone-loaded poly(D,L-lactic acid) microspheres embedded in poly(ethylene glycol)-poly(-caprolactone)-poly(ethylene glycol) hydrogel for orthopedic tissue engineering. J. Biomater. Appl. 2013, 28, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Liu, S.; Zheng, W.L.; Xiu, Z.L.; Zhang, Q. Dendrimer complexed hydrogel for local delivery of PTX to inhibit recurrence of breast cancer. J. Control. Release 2015, 213, e54. [Google Scholar] [CrossRef]
- Zhong, S.; Yung, L.Y. Enhanced biological stability of collagen with incorporation of PAMAM dendrimer. J. Biomed. Mater. Res. A 2009, 91, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.D.; Luckanagul, J.A.; Allen, A.; Ramaboli, M.; Campbell, E.; West, D.; Maturavongsadit, P.; Brummett, K.; Wang, Q. Synthesis of PAMAM dendrimer-based fast cross-linking hydrogel for biofabrication. J. Biomater. Sci. Polym. Ed. 2015, 26, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Q.; Zhang, H.; Yang, S.; Jia, X.R. A Novel poly(amido amine)-dendrimer-based hydrogel as a mimic for the extracellular matrix. Adv. Mater. 2014, 26, 4163–4167. [Google Scholar] [CrossRef] [PubMed]
- Winnicka, K.; Wroblewska, M.; Wieczorek, P.; Sacha, P.T.; Tryniszewska, E. Hydrogel of ketoconazole and pamam dendrimers: Formulation and antifungal activity. Molecules 2012, 17, 4612–4624. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.N.; Yuan, Q.; Yang, H. Synthesis and characterization of photocurable polyamidoamine dendrimer hydrogels as a versatile platform for tissue engineering and drug delivery. Biomacromolecules 2010, 11, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Din, F.U.; Mustapha, O.; Kim, D.W.; Rashid, R.; Park, J.H.; Choi, J.Y.; Ku, S.K.; Yong, C.S.; Kim, J.O.; Choi, H.G. Novel dual-reverse thermosensitive solid lipid nanoparticle-loaded hydrogel for rectal administration of flurbiprofen with improved bioavailability and reduced initial burst effect. Eur. J. Pharm. Biopharm. 2015, 94, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.W.; Vecchio, D.; Li, J.M.; Zhu, J.Y.; Zhang, Q.Z.; Fu, V.; Li, J.Y.; Thamphiwatana, S.; Lu, D.N.; Zhang, L.F. Hydrogel containing nanoparticle-stabilized liposomes for topical antimicrobial delivery. ACS Nano 2014, 8, 2900–2907. [Google Scholar] [CrossRef] [PubMed]
- Wickremasinghe, N.C.; Kumar, V.A.; Hartgerink, J.D. Two-step self-assembly of liposome-multidomain peptide nanofiber hydrogel for time-controlled release. Biomacromolecules 2014, 15, 3587–3595. [Google Scholar] [CrossRef] [PubMed]
- El Kechai, N.; Bochot, A.; Huang, N.; Nguyen, Y.; Ferrary, E.; Agnely, F. Effect of liposomes on rheological and syringeability properties of hyaluronic acid hydrogels intended for local injection of drugs. Int. J. Pharm. 2015, 487, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, L.K.; Bora, M.; Chan, P.N.P.H.; Lipik, V.; Wong, T.T.L.; Venkatraman, S.S. Hyaluronic acid-based nanocomposite hydrogels for ocular drug delivery applications. J. Biomed. Mater. Res. A 2014, 102, 3056–3065. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Jiang, D.H.; Wang, Z.; Wu, G.Z.; Miao, L.Y.; Huang, L.X. Intra-articular delivery of liposomal celecoxib-hyaluronate combination for the treatment of osteoarthritis in rabbit model. Int. J. Pharm. 2013, 441, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Lajavardi, L.; Camelo, S.; Agnely, F.; Luo, W.; Goldenberg, B.; Naud, M.C.; Behar-Cohen, F.; de Kozak, Y.; Bochot, A. New formulation of vasoactive intestinal peptide using liposomes in hyaluronic acid gel for uveitis. J. Control. Release 2009, 139, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Shao, H.; Liu, H. Preparation and characterization of film-forming raspberry-like polymer/silica nanocomposites via soap-free emulsion polymerization and the sol–gel process. Colloid Polym. Sci. 2012, 291, 1181–1190. [Google Scholar] [CrossRef]
- Suri, A.; Campos, R.; Rackus, D.G.; Spiller, N.J.S.; Richardson, C.; Palsson, L.O.; Kataky, R. Liposome-doped hydrogel for implantable tissue. Soft Matter 2011, 7, 7071–7077. [Google Scholar] [CrossRef]
- Popescu, M.T.; Mourtas, S.; Pampalakis, G.; Antimisiaris, S.G.; Tsitsilianis, C. pH-responsive hydrogel/liposome soft nanocomposites for tuning drug release. Biomacromolecules 2011, 12, 3023–3030. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Chen, L.J.; Tan, L.W.; Zhao, Q.; Luo, F.; Wei, Y.Q.; Qian, Z.Y. PEG-PCL based micelle hydrogels as oral docetaxel delivery systems for breast cancer therapy. Biomaterials 2014, 35, 6972–6985. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.Y.; Wu, Q.J.; Wang, Y.J.; Zhang, D.D.; Luo, F.; Zhao, X.; Wei, Y.Q.; Qian, Z.Y. A biodegradable hydrogel system containing curcumin encapsulated in micelles for cutaneous wound healing. Biomaterials 2013, 34, 6377–6387. [Google Scholar] [CrossRef] [PubMed]
- Jin, N.X.; Morin, E.A.; Henn, D.M.; Cao, Y.; Woodcock, J.W.; Tang, S.C.; He, W.; Zhao, B. Agarose hydrogels embedded with pH-responsive diblock copolymer micelles for triggered release of substances. Biomacromolecules 2013, 14, 2713–2723. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, C.; Khan, M.; Liu, S.Q.; Hedrick, J.L.; Yang, Y.Y.; Ee, P.L.R. Nanostructured PEG-based hydrogels with tunable physical properties for gene delivery to human mesenchymal stem cells. Biomaterials 2012, 33, 6533–6541. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Mukai, S.A.; Sawada, S.I.; Sasaki, Y.; Akiyoshi, K. Nanogel tectonic porous gel loading biologics, nanocarriers, and cells for advanced scaffold. Biomaterials 2015, 37, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Meid, J.; Friedrich, T.; Tieke, B.; Lindner, P.; Richtering, W. Composite hydrogels with temperature sensitive heterogeneities: Influence of gel matrix on the volume phase transition of embedded poly-(N-isopropylacrylamide) microgels. Phys. Chem. Chem. Phys. 2011, 13, 3039–3047. [Google Scholar] [CrossRef] [PubMed]
- Sekine, Y.; Moritani, Y.; Ikeda-Fukazawa, T.; Sasaki, Y.; Akiyoshi, K. A hybrid hydrogel biomaterial by nanogel engineering: Bottom-up design with nanogel and liposome building blocks to develop a multidrug delivery system. Adv. Healthc. Mater. 2012, 1, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Molinos, M.; Carvalho, V.; Silva, D.M.; Gama, F.M. Development of a hybrid dextrin hydrogel encapsulating dextrin nanogel as protein delivery system. Biomacromolecules 2012, 13, 517–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivakumaran, D.; Maitland, D.; Hoare, T. Injectable microgel-hydrogel composites for prolonged small-molecule drug delivery. Biomacromolecules 2011, 12, 4112–4120. [Google Scholar] [CrossRef] [PubMed]
- Hirakura, T.; Yasugi, K.; Nemoto, T.; Sato, M.; Shimoboji, T.; Aso, Y.; Morimoto, N.; Akiyoshi, K. Hybrid hyaluronan hydrogel encapsulating nanogel as a protein nanocarrier: New system for sustained delivery of protein with a chaperone-like function. J. Control. Release 2010, 142, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Liu, H.; Xie, Y.J.; Fang, C.; Yang, H.Y. Thermal-responsive self-healing hydrogel based on hydrophobically modified chitosan and vesicle. Colloid Polym. Sci. 2013, 291, 1749–1758. [Google Scholar] [CrossRef]
- Litvinchuk, S.; Lu, Z.; Rigler, P.; Hirt, T.D.; Meier, W. Calcein release from polymeric vesicles in blood plasma and PVA hydrogel. Pharm. Res. 2009, 26, 1711–1717. [Google Scholar] [CrossRef] [PubMed]
- Marianecci, C.; Carafa, M.; di Marzio, L.; Rinaldi, F.; di Meo, C.; Alhaique, F.; Matricardi, P.; Coviello, T. A new vesicle-loaded hydrogel system suitable for topical applications: Preparation and characterization. J. Pharm. Pharm. Sci. 2011, 14, 336–346. [Google Scholar]
- Jeon, O.; Wolfson, D.W.; Alsberg, E. In situ formation of growth-factor-loaded coacervate microparticle-embedded hydrogels for directing encapsulated stem cell fate. Adv. Mater. 2015, 27, 2216–2223. [Google Scholar] [CrossRef] [PubMed]
- Luckanagul, J.; Lee, L.A.; Nguyen, Q.L.; Sitasuwan, P.; Yang, X.M.; Shazly, T.; Wang, Q. Porous alginate hydrogel functionalized with virus as three-dimensional scaffolds for bone differentiation. Biomacromolecules 2012, 13, 3949–3958. [Google Scholar] [CrossRef] [PubMed]
- Lampe, K.J.; Kern, D.S.; Mahoney, M.J.; Bjugstad, K.B. The administration of BDNF and GDNF to the brain via PLGA microparticles patterned within a degradable PEG-based hydrogel: Protein distribution and the glial response. J. Biomed. Mater. Res. A 2011, 96, 595–607. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Liu, C.; Zhu, J.; Pochan, D.J.; Jia, X. Hybrid, elastomeric hydrogels crosslinked by multifunctional block copolymer micelles. Soft Matter 2010, 6, 5293–5297. [Google Scholar] [CrossRef] [PubMed]
- Weiner, A.L.; Carpentergreen, S.S.; Soehngen, E.C.; Lenk, R.P.; Popescu, M.C. Liposome–collagen gel matrix: A novel sustained drug delivery system. J. Pharm. Sci. 1985, 74, 922–925. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.M.; Marques, C.S.; Oliveira, R.S.; Coelho, P.B.; Costa, P.C.; Ferreira, D.C. Sustained drug release by contact lenses for glaucoma treatment: A review. J. Control. Release 2015, 202, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Gulsen, D.; Chauhan, A. Dispersion ofmicroemulsion drops in HEMA hydrogel: A potential ophthalmic drug delivery vehicle. Int. J. Pharm. 2005, 292, 95–117. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-H.; Keller, P. Stimuli-responsive polymer vesicles. Soft Matter 2009, 5, 927–937. [Google Scholar] [CrossRef]
- Mart, R.J.; Liem, K.P.; Webb, S.J. Magnetically-controlled release from hydrogel-supported vesicle assemblies. Chem. Commun. 2009, 2009, 2287–2289. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Son, J.Y.; Choi, J.H.; Kim, I.G.; Lee, Y.; Lee, J.Y.; Park, K.D. Macro/nano-gel composite as an injectable and bioactive bulking material for the treatment of urinary incontinence. Biomacromolecules 2014, 15, 1979–1984. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.; Al-Zaid, B.; El-Hashim, A.Z.; Chandrasekhar, B.; Attur, S.; Yousif, M.H.M.; Benter, I.F. Cationic polyamidoamine dendrimers as modulators of EGFR signaling in vitro and in vivo. PLoS ONE 2015, 10, e0132215. [Google Scholar] [CrossRef] [PubMed]
- Gillies, E.R.; Frechet, J.M. Dendrimers and dendritic polymers in drug delivery. Drug Discov. Today 2005, 10, 35–43. [Google Scholar] [CrossRef]
- Söntjens, S.H.; Nettles, D.L.; Carnahan, M.A.; Setton, L.A.; Grinstaff, M.W. Biodendrimer-based hydrogel scaffolds for cartilage tissue repair. Biomacromolecules 2006, 7, 310–316. [Google Scholar]
- Zhang, H.; Patel, A.; Gaharwar, A.K.; Mihaila, S.M.; Iviglia, G.; Mukundan, S.; Bae, H.; Yang, H.; Khademhosseini, A. Hyperbranched polyester hydrogels with controlled drug release and cell adhesion properties. Biomacromolecules 2013, 14, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of Polymer Hydrogels and Nanoparticulate Systems for Biomedical and Pharmaceutical Applications. Nanomaterials 2015, 5, 2054-2130. https://doi.org/10.3390/nano5042054
Zhao F, Yao D, Guo R, Deng L, Dong A, Zhang J. Composites of Polymer Hydrogels and Nanoparticulate Systems for Biomedical and Pharmaceutical Applications. Nanomaterials. 2015; 5(4):2054-2130. https://doi.org/10.3390/nano5042054
Chicago/Turabian StyleZhao, Fuli, Dan Yao, Ruiwei Guo, Liandong Deng, Anjie Dong, and Jianhua Zhang. 2015. "Composites of Polymer Hydrogels and Nanoparticulate Systems for Biomedical and Pharmaceutical Applications" Nanomaterials 5, no. 4: 2054-2130. https://doi.org/10.3390/nano5042054
APA StyleZhao, F., Yao, D., Guo, R., Deng, L., Dong, A., & Zhang, J. (2015). Composites of Polymer Hydrogels and Nanoparticulate Systems for Biomedical and Pharmaceutical Applications. Nanomaterials, 5(4), 2054-2130. https://doi.org/10.3390/nano5042054