Facile Synthesis of Gd-Functionalized Gold Nanoclusters as Potential MRI/CT Contrast Agents
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation of Gd-Au NCs
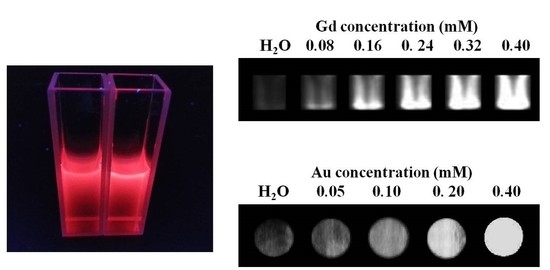
2.2. Characterization of Gd-Au NCs
2.3. MRI/CT in Vitro
2.4. In Vitro Cytotoxicity
3. Materials and Methods
3.1. Materials
3.2. Preparation of Gd-Au NCs
3.3. Characterization of Gd-AuNCs
3.4. Relaxometry and MRI in Vitro
3.5. In Vitro Cytotoxicity
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cherry, S.R. Multimodality imaging: Beyond PET/CT and SPECT/CT. Semi. Nucl. Med. 2009, 39, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Culver, J.; Akers, W.; Achilefu, S. Multimodality molecular imaging with combined optical and SPECT/PET modalities. J. Nucl. Med. 2008, 49, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.K.; Chan, P.S.; Fan, S.; Kwan, S.M.; Yeung, K.L.; Wáng, Y.X.J.; Chow, A.H.L.; Wu, E.X.; Baum, L. Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer’s disease mice using magnetic resonance imaging (MRI). Biomaterials 2015, 44, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Louie, A. Multimodality imaging probes: Design and challenges. Chem. Rev. 2010, 110, 3146–3195. [Google Scholar] [CrossRef] [PubMed]
- Brammer, M.J.; Bullmore, E.T.; Simmons, A.; Williams, S.C.R.; Grasby, P.M.; Howard, R.J.; Woodruff, P.W.R.; Rabe-Hesketh, S. Generic brain activation mapping in functional magnetic resonance imaging: A nonparametric approach. Magn. Reson. Imag. 1997, 15, 763–770. [Google Scholar] [CrossRef]
- Weissleder, R.; Pittet, M.J. Imaging in the era of molecular oncology. Nature 2008, 452, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Taylor-Pashow, K.M.; Rocca, J.D.; Lin, W. Mesoporous silica nanoparticles with co-condensed gadolinium chelates for multimodal imaging. Nanomaterials 2011, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Park, W.; Hu, J.; Bae, Y.H.; Na, K. A cancer-recognizable MRI contrast agents using pH-responsive polymeric micelle. Biomaterials 2014, 35, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhu, L.; Yu, M.; Feng, F.; An, L.; Xing, C.; Wang, S. Gadolinium (III) chelated conjugated polymer as a potential MRI contrast agent. Polymer 2010, 51, 1336–1340. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, T.; Xiao, Y.; Yu, D.; Zhang, N. Hyaluronic Acid-Chitosan Nanoparticles to Deliver Gd-DTPA for MR Cancer Imaging. Nanomaterials 2015, 5, 1379–1396. [Google Scholar] [CrossRef]
- Yang, W.; Guo, W.; Gong, X.; Zhang, B.; Wang, S.; Chen, N.; Yang, W.; Tu, Y.; Fang, X.; Chang, J. Facile Synthesis of Gd–Cu–In–S/ZnS Bimodal Quantum Dots with Optimized Properties for Tumor Targeted Fluorescence/MR In Vivo Imaging. ACS Appl. Mater. Inter. 2015, 7, 18759–18768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Jin, H.; Li, Y.; Chen, B.; Liu, S.; Shi, D. Bioinspired synthesis of gadolinium-based hybrid nanoparticles as MRI blood pool contrast agents with high relaxivity. J. Mater. Chem. 2012, 22, 14494–14501. [Google Scholar] [CrossRef]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Brenner, D.J.; Hall, E.J. Computed tomography—An increasing source of radiation exposure. New Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Tu, Y.; Qin, S.; Li, Y.; Zhou, J.; Chen, N.; Liu, Q.; Zhang, B. Gold nanoclusters as contrast agents for fluorescent and X-ray dual-modality imaging. J. Colloid Interface Sci. 2012, 372, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.E.; Long, N.J. Two is better than one’-probes for dual-modality molecular imaging. Chem. Commun. 2009, 3511–3524. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Chen, X. Dual-modality probes for in vivo molecular imaging. Mol. Imaging 2009, 8, 87–100. [Google Scholar] [PubMed]
- Misri, R. Multimodality imaging. Future Med. 2013, 162–176. [Google Scholar]
- Darder, M.; Aranda, P.; Ruiz-Hitzky, E. Bionanocomposites: A new concept of ecological, bioinspired, and functional hybrid materials. Adv. Mater. 2007, 19, 1309–1319. [Google Scholar] [CrossRef]
- Tanaka, M.; Sato, K.; Kitakami, E.; Kobayashi, S.; Hoshiba, T.; Fukushima, K. Design of biocompatible and biodegradable polymers based on intermediate water concept. Polymer J. 2015, 47, 114–121. [Google Scholar] [CrossRef]
- Xie, J.; Zheng, Y.; Ying, J.Y. Protein-directed synthesis of highly fluorescent gold nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ye, F.; Fang, T.; Niu, W.; Liu, P.; Min, X.; Li, X. Bovine serum albumin-directed synthesis of biocompatible CdSe quantum dots and bacteria labeling. J. Colloid Interface Sci. 2011, 355, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fu, Y.Y.; Zhang, X.; Yu, C.; Zhao, Y.; Sun, S.K. BSA-directed synthesis of CuS nanoparticles as a biocompatible photothermal agent for tumor ablation in vivo. Dalton Trans. 2015, 44, 13112–13118. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Cai, P.; Yang, W.; Xue, J.; Gao, L.; Liu, R.; Wang, Y.; Zhao, Y.; He, X.; Zhao, L.; et al. Ultrasmall [(64)Cu]Cu Nanoclusters for Targeting Orthotopic Lung Tumors Using Accurate Positron Emission Tomography Imaging. ACS Nano 2015, 9, 4976–4986. [Google Scholar] [CrossRef] [PubMed]
- Isab, A.A.; Hormann, A.L.; Hill, D.T.; Griswold, D.E.; DiMartino, M.J.; ShawIII, C.F. Bis(triethylphosphine)gold(I) chloride: Ionization in aqueous solution, reduction in vitro of the external and internal disulfide bonds of bovine serum albumin and antiarthritic activity. Inorg. Chem. 1989, 28, 1321–1326. [Google Scholar] [CrossRef]
- Chen, H.; Li, S.; Li, B.; Ren, X.; Li, S.; Mahounga, D.M.; Cui, S.; Gu, Y.; Achilefu, S. Folate-modified gold nanoclusters as near-infrared fluorescent probes for tumor imaging and therapy. Nanoscale 2012, 4, 6050–6064. [Google Scholar] [CrossRef] [PubMed]
- Arndt, B.; Noei, H.; Keller, T.F.; Müller, P.; Vonk, V.; Nenning, A.; Opitz, A.K.; Fleig, J.; Rütt, U.; Stierle, A. Structure and stability of Gd-doped CeO2 thin films on yttria-stabilized zirconia. Thin Solid Films 2016, 603, 56–61. [Google Scholar] [CrossRef]
- Sun, G.; Zhou, L.; Liu, Y.; Zhao, Z. Biocompatible Gd III-functionalized fluorescent gold nanoclusters for optical and magnetic resonance imaging. New J. Chem. 2013, 37, 1028–1035. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, X.; Tang, X.; Wu, C.; Zhou, Z.; Sun, C.; Deng, S.-L.; Ai, H.; Gao, J. A multiple gadolinium complex decorated fullerene as a highly sensitive T(1) contrast agent. Chem. Commun. 2015, 51, 4390–4393. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Wang, X.; Zhang, Z.; Zhang, H.; Liu, H.; Zhu, X.; Li, H.; Chi, X.; Yin, Z.; Gao, J. Real-Time Monitoring of Arsenic Trioxide Release and Delivery by Activatable T(1) Imaging. ACS Nano 2015, 9, 2749–2759. [Google Scholar] [CrossRef] [PubMed]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium(III) chelates as MRI contrast agents: Structure, dynamics, and applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef] [PubMed]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, W.; Cui, S.; Chen, X.; Zhu, H.; Chen, B.; Cui, Z. Facile Synthesis of Gd-Functionalized Gold Nanoclusters as Potential MRI/CT Contrast Agents. Nanomaterials 2016, 6, 65. https://doi.org/10.3390/nano6040065
Le W, Cui S, Chen X, Zhu H, Chen B, Cui Z. Facile Synthesis of Gd-Functionalized Gold Nanoclusters as Potential MRI/CT Contrast Agents. Nanomaterials. 2016; 6(4):65. https://doi.org/10.3390/nano6040065
Chicago/Turabian StyleLe, Wenjun, Shaobin Cui, Xin Chen, Huanhuan Zhu, Bingdi Chen, and Zheng Cui. 2016. "Facile Synthesis of Gd-Functionalized Gold Nanoclusters as Potential MRI/CT Contrast Agents" Nanomaterials 6, no. 4: 65. https://doi.org/10.3390/nano6040065
APA StyleLe, W., Cui, S., Chen, X., Zhu, H., Chen, B., & Cui, Z. (2016). Facile Synthesis of Gd-Functionalized Gold Nanoclusters as Potential MRI/CT Contrast Agents. Nanomaterials, 6(4), 65. https://doi.org/10.3390/nano6040065