Gold Nanomaterial Uptake from Soil Is Not Increased by Arbuscular Mycorrhizal Colonization of Solanum Lycopersicum (Tomato)
Abstract
:1. Introduction
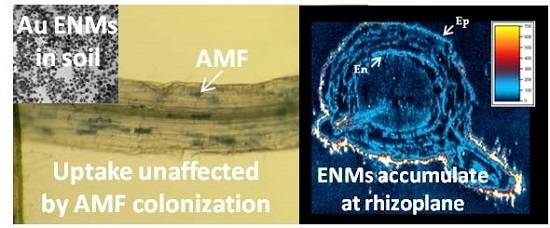
2. Results
3. Discussion
4. Materials and Methods
4.1. Nanomaterials
4.2. Plant Exposure
4.3. Au Extractions from Soil
4.4. Quantification of Mycorrhizal Colonization
4.5. Shoot Au Analysis
4.6. Root Sectioning and Analysis by LA-ICP-MS
4.7. Statistics
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CSIRO | Commonwealth Scientific and Industrial Research Organization |
| ENM | Engineered nanomaterial |
| AMF | Arbuscular mycorrhizal fungi |
| LFM | Laboratory fortified matrix |
| rmc | Reduced mycorrhizal colonization (tomato genotype) |
| LA-ICP-MS | Laser ablation inductively-coupled plasma mass spectrometry |
References
- Judy, J.D.; Unrine, J.M.; Rao, W.; Wirick, S.; Bertsch, P.M. Bioavailability of gold nanomaterials to plants: Importance of particle size and surface coating. Environ. Sci. Technol. 2012, 46, 8467–8474. [Google Scholar] [CrossRef] [PubMed]
- Sabo-Attwood, T.; Unrine, J.M.; Stone, J.W.; Murphy, C.J.; Ghoshroy, S.; Blom, D.; Bertsch, P.M.; Newman, L.A. Uptake, distribution and toxicity of gold nanoparticles in tobacco (Nicotiana xanthi) seedlings. Nanotoxicology 2011, 6, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Kurepa, J.; Paunesku, T.; Vogt, S.; Arora, H.; Rabatic, B.M.; Lu, J.; Wanzer, M.B.; Woloschak, G.E.; Smalle, J.A. Uptake and distribution of ultrasmall anatase TiO2 alizarin red S nanoconjugates in Arabidopsis thaliana. Nano Lett. 2010, 10, 2296–2302. [Google Scholar] [CrossRef] [PubMed]
- Colman, B.P.; Arnaout, C.L.; Anciaux, S.; Gunsch, C.K.; Hochella, M.F., Jr.; Kim, B.; Lowry, G.V.; McGill, B.M.; Reinsch, B.C.; Richardson, C.J. Low concentrations of silver nanoparticles in biosolids cause adverse ecosystem responses under realistic field scenario. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Du, W.; Sun, Y.; Ji, R.; Zhu, J.; Wu, J.; Guo, H. TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J. Environ. Monit. 2011, 13, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Judy, J.D.; Kirby, J.K.; Creamer, C.; McLaughlin, M.J.; Fiebiger, C.; Wright, C.; Cavagnaro, T.; Bertsch, P.M. Effects of silver sulfide nanomaterials on mycorrhizal colonization of tomato plants and soil microbial communities in biosolid-amended soil. Environ. Pollut. 2015, 206, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Judy, J.D.; McNear, D.; Chen, C.; Lewis, R.W.; Tsyusko, O.V.; Bertsch, P.M.; Rao, W.; Stegemeier, J.; Lowry, G.V.; McGrath, S.P. Nanomaterials in biosolids inhibit nodulation, shift microbial community composition, and result in increased metal uptake relative to bulk metals. Environ. Sci. Technol. 2015, 49, 8751–8758. [Google Scholar] [CrossRef] [PubMed]
- Doolette, C.L.; McLaughlin, M.J.; Kirby, J.K.; Navarro, D.A. Bioavailability of silver and silver sulfide nanoparticles to lettuce (Lactuca sativa): Effect of agricultural amendments on plant uptake. J. Hazard. Mater. 2015, 300, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Priester, J.H.; Ge, Y.; Mielke, R.D.; Horst, A.M.; Moritz, S.C.; Espinosa, K.; Gelb, J.; Walker, S.L.; Nisbet, R.M.; An, Y.; et al. Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. Proc. Natl. Acad. Sci. USA 2012, 109, E2451–E2456. [Google Scholar] [CrossRef] [PubMed]
- Judy, J.D.; Bertsch, P.M. Bioavailability, toxicity and fate of manufactured nanomaterials in terrestrial ecosystems. In Advances in Agronomy; Sparks, D., Ed.; Elsevier: Amsterdam, Netherlands, 2014; Volume 123, pp. 1–64. [Google Scholar]
- Watts-Williams, S.J.; Turney, T.W.; Patti, A.F.; Cavagnaro, T.R. Uptake of zinc and phosphorus by plants is affected by zinc fertiliser material and arbuscular mycorrhizas. Plant Soil 2014, 376, 165–175. [Google Scholar] [CrossRef]
- Feng, Y.; Cui, X.; He, S.; Dong, G.; Chen, M.; Wang, J.; Lin, X. The role of metal nanoparticles in influencing arbuscular mycorrhizal fungi effects on plant growth. Environ. Sci. Technol. 2013, 47, 9496–9504. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, M.D.; Treseder, K.K.; Atsatt, P.R. The brighter side of soils: Quantum dots track organic nitrogen through fungi and plants. Ecology 2009, 90, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Levard, C.; Judy, J.D.; Unrine, J.M.; Durenkamp, M.; Martin, B.; Jefferson, B.; Lowry, G.V. Fate of zinc oxide and silver nanoparticles in a pilot wastewater treatment plant and in processed biosolids. Environ. Sci. Technol. 2013, 48, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Chen, B.; Wang, Q.; Shi, X.; Xiao, Z.; Lin, J.; Fang, X. Carbon nanotubes as molecular transporters for walled plant cells. Nano Lett. 2009, 9, 1007–1010. [Google Scholar] [CrossRef] [PubMed]
- Geisler-Lee, J.; Wang, Q.; Yao, Y.; Zhang, W.; Geisler, M.; Li, K.; Huang, Y.; Chen, Y.; Komakov, A.; Ma, X. Phytotoxicity, accumulation and transport of silver nanoparticles by Arabidopsis thaliana. Nanotoxicology 2013, 7, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.J.; Herth, S. Plant nanotoxicology. Trends Plant Sci. 2011, 16, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Schwab, F.; Zhai, G.; Kern, M.; Turner, A.; Schnoor, J.L.; Wiesner, M.R. Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants-Critical review. Nanotoxicology 2015, 10, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Barker, S.J.; Stummer, G.; Gao, L.; Dispain, I.; O‘Connor, P.J.; Smith, S.E. A mutant in Lycopersicon esculentum Mill. with highly reduced VA mycorrhizal colonization: Isolation and preliminary characterization. Plant J. 1998, 15, 791–797. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circular. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Hall, G.E.; MacLaurin, A.I.; Garrett, R.G. Assessment of the 1 M NH4NO3 extraction protocol to identify mobile forms of Cd in soils. J. Geochem. Explor. 1998, 64, 153–159. [Google Scholar] [CrossRef]
- USEPA. Method 3051A: Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils; United States Environmental Protection Agency (USEPA): Washington, DC, USA, 2007. [Google Scholar]
- Vierheiling, H.; Coughlan, A.P.; Wyss, U.; Piche, Y. Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl. Environ. Microbiol. 1998, 64, 5004–5007. [Google Scholar]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Childress, C.J.O.; Foreman, W.T.; Connor, B.F.; Maloney, T.J. New Reporting Procedures Based on Long-Term Method Detection Levels and Some Considerations for Interpretations of Water-Quality Data Provided by the US Geological Survey (No. 99–193); National Water-Quality Laboratory, Ed.; US Department of the Interior, US Geological Survey: Reston, VA, USA, 1999. [Google Scholar]
- Muller, W.; Shelley, M.; Miller, P.; Broude, S. Initial performance metrics of a new custom-designed ArF excimer LA-ICPMS system coupled to a two-volume laser-ablation cell. J. Anal. At. Spectrom. 2008, 24, 209–214. [Google Scholar] [CrossRef]
- Paton, C.; Hellstrom, J.; Paul, B.; Woodhead, J.; Hergt, J. Iolite: Freeware for the visualisation and processing of mass spectrometric data. J. Anal. At. Spectrom. 2011, 26, 2508–2518. [Google Scholar] [CrossRef]
- Whitley, A.R.; Levard, C.; Oostveen, E.; Bertsch, P.M.; Matocha, C.J.; von der Kammer, F.; Unrine, J.M. Behavior of Ag nanoparticles in soil: Effects of particle surface coating, aging and sewage sludge amendment. Environ. Pollut. 2013, 182, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.P.; Petersen, E.; Bissegger, S.; Koch, I.; Zhang, J.; Reimer, K.J.; Rehmann, L.; Slawson, R.M.; Legge, R.L.; O’Carroll, D.M. Effect of gold nanoparticles and ciprofloxacin on microbial catabolism: A community-based approach. Environ. Toxicol. Chem. 2014, 33, 44–51. [Google Scholar] [CrossRef] [PubMed]



| Treatment | Z-Average Diameter (nm) | Polydispersivity Index | TEM Diameter (nm) | TEM Range (nm) | Zeta Potential (mv ± zeta deviation) |
|---|---|---|---|---|---|
| Au ENMs | 26.5 ± 4.9 | 0.29 ± 0.1 | 9.9 ± 2.7 | 3.5–17.8 | −58.0 ± 5.9 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Judy, J.D.; Kirby, J.K.; McLaughlin, M.J.; Cavagnaro, T.; Bertsch, P.M. Gold Nanomaterial Uptake from Soil Is Not Increased by Arbuscular Mycorrhizal Colonization of Solanum Lycopersicum (Tomato). Nanomaterials 2016, 6, 68. https://doi.org/10.3390/nano6040068
Judy JD, Kirby JK, McLaughlin MJ, Cavagnaro T, Bertsch PM. Gold Nanomaterial Uptake from Soil Is Not Increased by Arbuscular Mycorrhizal Colonization of Solanum Lycopersicum (Tomato). Nanomaterials. 2016; 6(4):68. https://doi.org/10.3390/nano6040068
Chicago/Turabian StyleJudy, Jonathan D., Jason K. Kirby, Mike J. McLaughlin, Timothy Cavagnaro, and Paul M. Bertsch. 2016. "Gold Nanomaterial Uptake from Soil Is Not Increased by Arbuscular Mycorrhizal Colonization of Solanum Lycopersicum (Tomato)" Nanomaterials 6, no. 4: 68. https://doi.org/10.3390/nano6040068
APA StyleJudy, J. D., Kirby, J. K., McLaughlin, M. J., Cavagnaro, T., & Bertsch, P. M. (2016). Gold Nanomaterial Uptake from Soil Is Not Increased by Arbuscular Mycorrhizal Colonization of Solanum Lycopersicum (Tomato). Nanomaterials, 6(4), 68. https://doi.org/10.3390/nano6040068