The Influence of Fluorination on Nano-Scale Phase Separation and Photovoltaic Performance of Small Molecular/PC71BM Blends
Abstract
:1. Introduction
2. Results and discussions
2.1. Synthesis
2.2. Optical Properties
2.3. Cyclic Voltammetry
2.4. Photovoltaic Properties
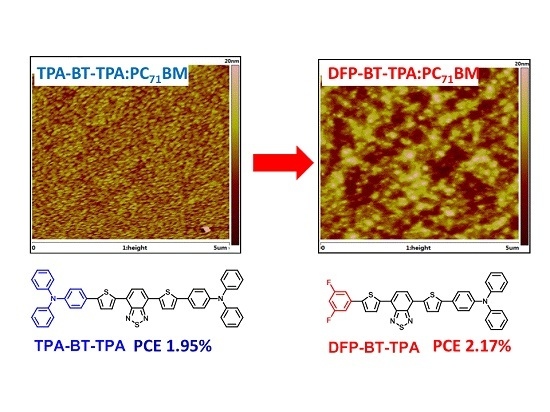
2.5. Film Morphology
3. Experimental Section
3.1. Materials
3.2. Measurements and Characterization
3.3. Fabrication and Characterization of SCLC
3.4. Fabrication and Characterization of PSCs
3.5. General Procedure for the Synthesis of Small Molecules
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liang, Y.; Xu, Z.; Xia, J.; Tsai, S.-T.; Wu, Y.; Li, G.; Ray, C.; Yu, L. For the Bright Future—Bulk Heterojunction Polymer Solar Cells with Power Conversion Efficiency of 7.4%. Adv. Mater. 2010, 22, E135–E138. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zheng, T.; Wu, Q.; Schneider, A.M.; Zhao, D.; Yu, L. Recent Advances in Bulk Heterojunction Polymer Solar Cells. Chem. Rev. 2015, 115, 12666–12731. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cao, Y. Development of Novel Conjugated Donor Polymers for High-Efficiency Bulk-Heterojunction Photovoltaic Devices. Acc. Chem. Res. 2009, 42, 1709–1718. [Google Scholar] [CrossRef] [PubMed]
- Li, Y. Molecular Design of Photovoltaic Materials for Polymer Solar Cells: Toward Suitable Electronic Energy Levels and Broad Absorption. Acc. Chem. Res. 2012, 45, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Li, Y.; Zhan, X. Small molecule semiconductors for high-efficiency organic photovoltaics. Chem. Soc. Rev. 2012, 41, 4245–4272. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Guo, X.; Min, J.; Guo, B.; Cheng, X.; Zhang, M.; Brabec, C.J.; Li, Y. High-Performance Organic Solar Cells Based on a Small Molecule with Alkylthio-Thienyl-Conjugated Side Chains without Extra Treatments. Adv. Mater. (Deerfield Beach Fla.) 2015, 27, 7469–7475. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yi, C.; Wang, K.; Yang, Y.L.; Bhatta, R.S.; Tsige, M.; Xiao, S.Y.; Gong, X. Single-Junction Polymer Solar Cells with Over 10% Efficiency by a Novel Two-Dimensional Donor-Acceptor Conjugated Copolymer. ACS Appl. Mater. Interfaces 2015, 7, 4928–4935. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Fan, H.; Liu, Y.; Hu, W.; Li, Y.; Zhan, X. A Solution-Processable Star-Shaped Molecule for High-Performance Organic Solar Cells. Adv. Mater. 2011, 23, 1554–1557. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Welch, G.C.; Leong, W.L.; Takacs, C.J.; Bazan, G.C.; Heeger, A.J. Solution-processed small-molecule solar cells with 6.7% efficiency. Nat. Mater. 2012, 11, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kan, B.; Liu, F.; Long, G.; Wan, X.; Chen, X.; Zuo, Y.; Ni, W.; Zhang, H.; Li, M.; et al. Small-molecule solar cells with efficiency over 9%. Nat. Photonics 2015, 9, 35–41. [Google Scholar] [CrossRef]
- Kan, B.; Zhang, Q.; Li, M.; Wan, X.; Ni, W.; Long, G.; Wang, Y.; Yang, X.; Feng, H.; Chen, Y. Solution-Processed Organic Solar Cells Based on Dialkylthiol-Substituted Benzodithiophene Unit with Efficiency near 10%. J. Am. Chem. Soc. 2014, 136, 15529–15532. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Li, M.; Kan, B.; Zuo, Y.; Zhang, Q.; Long, G.; Feng, H.; Wan, X.; Chen, Y. Open-circuit voltage up to 1.07 V for solution processed small molecule based organic solar cells. Organic Electron. 2014, 15, 2285–2294. [Google Scholar] [CrossRef]
- Carsten, B.; Szarko, J.M.; Son, H.J.; Wang, W.; Lu, L.; He, F.; Rolczynski, B.S.; Lou, S.J.; Chen, L.X.; Yu, L. Examining the Effect of the Dipole Moment on Charge Separation in Donor-Acceptor Polymers for Organic Photovoltaic Applications. J. Am. Chem. Soc. 2011, 133, 20468–20475. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Z.; Ling, M.M.; Mo, X.; Shi, M.M.; Wang, M.; Bao, Z. Air stable n-channel organic semiconductors for thin film transistors based on fluorinated derivatives of perylene diimides. Chem. Mater. 2007, 19, 816–824. [Google Scholar] [CrossRef]
- Li, K.; Li, Z.; Feng, K.; Xu, X.; Wang, L.; Peng, Q. Development of Large Band-Gap Conjugated Copolymers for Efficient Regular Single and Tandem Organic Solar Cells. J. Am. Chem. Soc. 2013, 135, 13549–13557. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.C.; Huang, Z.; Ashraf, R.S.; Smith, J.; D’Angelo, P.; Watkins, S.E.; Anthopoulos, T.D.; Durrant, J.R.; McCulloch, I. Silaindacenodithiophene-Based Low Band Gap Polymers—The Effect of Fluorine Substitution on Device Performances and Film Morphologies. Adv. Funct. Mater. 2012, 22, 1663–1670. [Google Scholar] [CrossRef]
- Shewmon, N.T.; Watkins, D.L.; Galindo, J.F.; Zerdan, R.B.; Chen, J.; Keum, J.; Roitberg, A.E.; Xue, J.; Castellano, R.K. Enhancement in Organic Photovoltaic Efficiency through the Synergistic Interplay of Molecular Donor Hydrogen Bonding and π-Stacking. Adv. Funct. Mater. 2015, 25, 5166–5177. [Google Scholar] [CrossRef]
- Wang, L.; Yin, L.; Ji, C.; Li, Y. Tuning the photovoltaic performance of BT-TPA chromophore based solution-processed solar cells through molecular design incorporating of bithiophene unit and fluorine-substitution. Dyes Pigments 2015, 118, 37–44. [Google Scholar] [CrossRef]
- Sonar, P.; Ng, G.-M.; Lin, T.T.; Dodabalapur, A.; Chen, Z.-K. Solution processable low bandgap diketopyrrolopyrrole (DPP) based derivatives: Novel acceptors for organic solar cells. J. Mater. Chem. 2010, 20, 3626–3636. [Google Scholar] [CrossRef]
- Cui, R.; Fan, L.; Yuan, J.; Jiang, L.; Chen, G.; Ding, Y.; Shen, P.; Li, Y.; Zou, Y. Effect of fluorination on the performance of poly(thieno[2,3-f]benzofuran-co-benzothiadiazole) derivatives. RSC Adv. 2015, 5, 30145–30152. [Google Scholar] [CrossRef]
- Guo, S.; Ning, J.; Koerstgens, V.; Yao, Y.; Herzig, E.M.; Roth, S.V.; Mueller-Buschbaum, P. The Effect of Fluorination in Manipulating the Nanomorphology in PTB7:PC71BM Bulk Heterojunction Systems. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Jo, J.W.; Jung, J.W.; Jung, E.H.; Ahn, H.; Shin, T.J.; Jo, W.H. Fluorination on both D and A units in D-A type conjugated copolymers based on difluorobithiophene and benzothiadiazole for highly efficient polymer solar cells. Energy Environ. Sci. 2015, 8, 2427–2434. [Google Scholar] [CrossRef]
- Kim, H.G.; Kang, B.; Ko, H.; Lee, J.; Shin, J.; Cho, K. Synthetic Tailoring of Solid-State Order in Diketopyrrolopyrrole-Based Copolymers via Intramolecular Noncovalent Interactions. Chem. Mater. 2015, 27, 829–838. [Google Scholar] [CrossRef]
- Wang, J.-L.; Wu, Z.; Miao, J.-S.; Liu, K.-K.; Chang, Z.-F.; Zhang, R.-B.; Wu, H.-B.; Cao, Y. Solution-Processed Diketopyrrolopyrrole-Containing Small-Molecule Organic Solar Cells with 7.0% Efficiency: In-Depth Investigation on the Effects of Structure Modification and Solvent Vapor Annealing. Chem. Mater. 2015, 27, 4338–4348. [Google Scholar] [CrossRef]
- Cho, A.; Kim, Y.; Song, C.E.; Moon, S.-J.; Lim, E. Synthesis and Characterization of Fluorinated Benzothiadiazole-Based Small Molecules for Organic Solar Cells. Sci. Adv. Mater. 2014, 6, 2411–2415. [Google Scholar] [CrossRef]
- Paek, S.; Cho, N.; Song, K.; Jun, M.J.; Lee, J.K.; Ko, J. Efficient Organic Semiconductors Containing Fluorine-Substituted Benzothiadiazole for Solution-Processed Small Molecule Organic Solar Cells. J. Phys. Chem. C 2012, 116, 23205–23213. [Google Scholar] [CrossRef]
- Cho, N.; Song, K.; Lee, J.K.; Ko, J. Facile Synthesis of Fluorine-Substituted Benzothiadiazole-Based Organic Semiconductors and Their Use in Solution-Processed Small-Molecule Organic Solar Cells. Chem. Eur. J. 2012, 18, 11433–11439. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Yang, W.; Eom, S.H.; Lee, S.-H. Synthesis and characterization of triphenylamine flanked thiazole-based small molecules for high performance solution processed organic solar cells. Organic Electron. 2012, 13, 273–282. [Google Scholar] [CrossRef]
- Li, Z.; Dong, Q.; Li, Y.; Xu, B.; Deng, M.; Pei, J.; Zhang, J.; Chen, F.; Wen, S.; Gao, Y.; Tian, W. Design and synthesis of solution processable small molecules towards high photovoltaic performance. J. Mater. Chem. 2011, 21, 2159–2168. [Google Scholar] [CrossRef]
- Lin, Y.; Cheng, P.; Li, Y.; Zhan, X. A 3D star-shaped non-fullerene acceptor for solution-processed organic solar cells with a high open-circuit voltage of 1.18 V. Chem. Commun. 2012, 48, 4773–4775. [Google Scholar] [CrossRef] [PubMed]
- Vijay Kumar, C.; Cabau, L.; Koukaras, E.N.; Sharma, G.D.; Palomares, E. Efficient solution processed D1-A-D2-A-D1 small molecules bulk heterojunction solar cells based on alkoxy triphenylamine and benzo[1,2-b:4,5-b′]thiophene units. Organic Electron. 2015, 26, 36–47. [Google Scholar] [CrossRef]
- Patil, H.; Chang, J.; Gupta, A.; Bilic, A.; Wu, J.; Sonar, P.; Bhosale, S.V. Isoindigo-Based Small Molecules with Varied Donor Components for Solution-Processable Organic Field Effect Transistor Devices. Molecules 2015, 20, 17362–17377. [Google Scholar] [CrossRef] [PubMed]
- Bagde, S.S.; Park, H.; Yang, S.-N.; Jin, S.-H.; Lee, S.-H. Diketopyrrolopyrrole-based narrow band gap donors for efficient solution-processed organic solar cells. Chem. Phys. Lett. 2015, 630, 37–43. [Google Scholar] [CrossRef]
- Mikroyannidis, J.A.; Stylianakis, M.M.; Suresh, P.; Balraju, P.; Sharma, G.D. Low band gap vinylene compounds with triphenylamine and benzothiadiazole segments for use in photovoltaic cells. Organic Electron. 2009, 10, 1320–1333. [Google Scholar] [CrossRef]
- Tolman, C.A.; Seidel, W.C.; Gerlach, D.H. Triarylphosphine and ethylene complexes of zerovalent nickel, palladium, and platinum. J. Am. Chem. Soc. 1972, 94, 2669–2676. [Google Scholar] [CrossRef]
- Kato, S.-I.; Matsumoto, T.; Ishi-i, T.; Thiemann, T.; Shigeiwa, M.; Gorohmaru, H.; Maeda, S.; Yamashita, Y.; Mataka, S. Strongly red-fluorescent novel donor-π-bridge-acceptor-π-bridge-donor (D-π-A-π-D) type 2,1,3-benzothiadiazoles with enhanced two-photon absorption cross-sections. Chem. Commun. 2004, 20, 2342–2343. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Gendron, D.; Badrou-Aïch, R.; Najari, A.; Tao, Y.; Leclerc, M. A High-Mobility Low-Bandgap Poly(2,7-carbazole) Derivative for Photovoltaic Applications. Macromolecules 2009, 42, 2891–2894. [Google Scholar] [CrossRef]
- Huang, J.; Zhan, C.; Zhang, X.; Zhao, Y.; Lu, Z.; Jia, H.; Jiang, B.; Ye, J.; Zhang, S.; Tang, A.; et al. Solution-Processed DPP-Based Small Molecule that Gives High Photovoltaic Efficiency with Judicious Device Optimization. ACS Appl. Mater. Interfaces 2013, 5, 2033–2039. [Google Scholar] [CrossRef] [PubMed]
- Uhrich, C.; Schueppel, R.; Petrich, A.; Pfeiffer, M.; Leo, K.; Brier, E.; Kilickiran, P.; Baeuerle, P. Organic Thin-Film Photovoltaic Cells Based on Oligothiophenes with Reduced Bandgap. Adv. Funct. Mater. 2007, 17, 2991–2999. [Google Scholar] [CrossRef]
- Lu, Z.; Li, C.-H.; Du, C.; Gong, X.; Bo, Z.-S. 6,7-dialkoxy-2,3-diphenylquinoxaline based conjugated polymers for solar cells with high open-circuit voltage. Chin. J. Polym. Sci. 2013, 31, 901–911. [Google Scholar] [CrossRef]
- Shang, Y.; Hao, S.; Liu, J.; Tan, M.; Wang, N.; Yang, C.; Chen, G. Synthesis of Upconversion beta-NaYF4:Nd3+/Yb3+/Er3+ Particles with Enhanced Luminescent Intensity through Control of Morphology and Phase. Nanomaterials 2015, 5, 218–232. [Google Scholar] [CrossRef]







| Donor | λabs(S) (nm) a | λabs(F) (nm) b | λonset (nm) | Eg, opt(ev) | HOMO c | LUMO d | Tm (°C) | Tg (°C) |
|---|---|---|---|---|---|---|---|---|
| DFP-BT-DFP | 488 | 505 | 636 | 1.95 | −5.73 | −3.78 | 246.6 | 350 |
| DFP-BT-TPA | 525 | 530 | 643 | 1.93 | −5.34 | −3.41 | 248.8 | 450 |
| TPA-BT-TPA | 544 | 547 | 659 | 1.88 | −5.17 | −3.29 | 254.4 | 461 |
| DFP-DPP-DFP | 598 | 616, 663 | 715 | 1.73 | −5.59 | −3.86 | 216.9 | 374 |
| DFP-DPP-TPA | 619 | 583, 627 | 746 | 1.66 | −5.13 | −3.47 | 195.3 | 400 |
| TPA-DPP-TPA | 634 | 588, 656 | 760 | 1.63 | −5.07 | −3.44 | 217.3 | 408 |
| Donor Molecule | Voc (V) | Jsc (mA·cm−2) | FF | PCE (max/ave) a (%) | Thickness (nm) | SCLC (cm2·V−1·s−1) |
|---|---|---|---|---|---|---|
| DFP-BT-TPA | 0.90 | 6.12 | 0.39 | 2.17/2.08 | 76 | 3.81 × 10−4 |
| TPA-BT-TPA | 0.82 | 5.97 | 0.40 | 1.95/1.85 | 82 | 4.42 × 10−5 |
| DFP-DPP-TPA | 0.78 | 4.95 | 0.32 | 1.22/1.17 | 70 | 9.35 × 10−5 |
| TPA-DPP-TPA | 0.70 | 4.90 | 0.34 | 1.15/1.02 | 85 | 3.26× 10−5 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Z.; Liu, W.; Li, J.; Fang, T.; Li, W.; Zhang, J.; Feng, F.; Li, W. The Influence of Fluorination on Nano-Scale Phase Separation and Photovoltaic Performance of Small Molecular/PC71BM Blends. Nanomaterials 2016, 6, 80. https://doi.org/10.3390/nano6040080
Lu Z, Liu W, Li J, Fang T, Li W, Zhang J, Feng F, Li W. The Influence of Fluorination on Nano-Scale Phase Separation and Photovoltaic Performance of Small Molecular/PC71BM Blends. Nanomaterials. 2016; 6(4):80. https://doi.org/10.3390/nano6040080
Chicago/Turabian StyleLu, Zhen, Wen Liu, Jingjing Li, Tao Fang, Wanning Li, Jicheng Zhang, Feng Feng, and Wenhua Li. 2016. "The Influence of Fluorination on Nano-Scale Phase Separation and Photovoltaic Performance of Small Molecular/PC71BM Blends" Nanomaterials 6, no. 4: 80. https://doi.org/10.3390/nano6040080
APA StyleLu, Z., Liu, W., Li, J., Fang, T., Li, W., Zhang, J., Feng, F., & Li, W. (2016). The Influence of Fluorination on Nano-Scale Phase Separation and Photovoltaic Performance of Small Molecular/PC71BM Blends. Nanomaterials, 6(4), 80. https://doi.org/10.3390/nano6040080