A Gallium Oxide-Graphene Oxide Hybrid Composite for Enhanced Photocatalytic Reaction
Abstract
:1. Introduction
2. Experimental Section
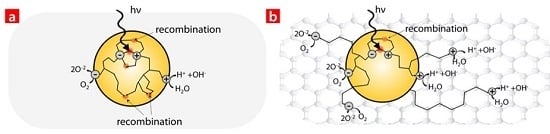
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, J.; Bai, H.; Wang, Y.; Liu, Z.; Zhang, X.; Sun, D.D. Self-assembling TiO2 nanorods on large graphene oxide sheets at a two-phase interface and their anti-recombination in photocatalytic applications. Adv. Funct. Mater. 2006, 20, 4175–4181. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Photocatalytic reduction of graphene oxide nanosheets on TiO2 thin film for photoinactivation of bacteria in solar light irradiation. J. Phys. Chem. C 2009, 113, 20214–20220. [Google Scholar] [CrossRef]
- Li, D.; Zhao, Y.; Wang, Q.; Yang, Y.; Zhang, Z. Enhanced biohydrogen production by accelerating the hydrolysis of macromolecular components of waste activated sludge using TiO2 photocatalysis as a pretreatment. Open J. Appl. Sci. 2013, 3. [Google Scholar] [CrossRef]
- Ohtani, B. Revisiting the fundamental physical chemistry in heterogeneous photocatalysis: Its thermodynamics and kinetics. Phys. Chem. Chem. Phys. 2014, 16, 1788–1797. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Park, W.K.; Hwang, T.M.; Yoon, D.H.; Yang, W.S.; Kang, J.W. Comparative evaluation of magnetite–graphene oxide and magnetite-reduced graphene oxide composite for As (III) and As (V) removal. J. Hazard. Mater. 2016, 304, 196–204. [Google Scholar] [CrossRef]
- Kudo, A.; Mikami, I. Photocatalytic activities and photophysical properties of Ga2–xInxO3 solid solution. J. Chem. Soc. 1998, 94, 2929–2932. [Google Scholar] [CrossRef]
- Luo, M.; Yao, W.; Huang, C.; Wu, Q.; Xu, Q. Shape effects of Pt nanoparticles on hydrogen production via Pt/CdS photocatalysts under visible light. J. Mater. Chem. A 2015, 3, 13884–13891. [Google Scholar] [CrossRef]
- Ben-Shahar, Y.; Scotognella, F.; Kriegel, I.; Moretti, L.; Cerullo, G.; Rabani, E.; Banin, U. Optimal metal domain size for photocatalysis with hybrid semiconductor-metal nanorods. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef]
- Girija, K.; Thirumalairajan, S.; Patra, A.K.; Mangalaraj, D.; Ponpandian, N.; Viswanathan, C. Organic additives assisted synthesis of mesoporous β-Ga2O3 nanostructures for photocatalytic dye degradation. Semicond. Sci. Technol. 2013, 28. [Google Scholar] [CrossRef]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Scaife, D.E. Oxide semiconductors in photoelectrochemical conversion of solar energy. Sol. Energy 1980, 25, 41–54. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, Z.; Xia, T.; Meng, H.; Low-Kam, C.; Liu, R.; Wang, M.; Lin, S.; Wang, X.; Liao, Y.-P.; et al. Use of metal oxide nanoparticle band gap to develop a predictive paradigm for oxidative stress and acute pulmonary inflammation. ACS Nano 2012, 6, 4349–4368. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.S.; Verma, A.; Peelaers, H.; Protasenko, V.; Rouvimov, S.; Xing, H.G.; Albrecht, M.; Seabaugh, A.; Haensch, W.; van de Walle, C.; et al. High-voltage field effect transistors with wide-bandgap β-Ga2O3 nanomembranes. Appl. Phys. Lett. 2014, 104. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Z.; Lin, C.; Lin, J. A simple method to synthesize β-Ga2O3 nanorods and their photoluminescence properties. J. Cryst. Growth 2005, 280, 99–106. [Google Scholar] [CrossRef]
- Ghazali, N.M.; Mahmood, M.R.; Yasui, K.; Hashim, A.M. Electrochemically deposited gallium oxide nanostructures on silicon substrates. Nanoscale Res. Lett. 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Robinson, J.T.; Diankov, G.; Dai, H. Nanocrystal growth on graphene with various degrees of oxidation. J. Am. Chem. Soc. 2010, 132, 3270–3271. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Chandkiram, G.; Prabhakar, S. Crystallization kinematics and dielectric behavior of (Ba, Sr) TiO3 borosilicate glass ceramics. New J. Glass Cerami. 2012, 2. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Han, K.I.; Lee, I.G.; Park, W.K.; Yoon, Y.; Yoo, C.S.; Yang, W.S.; Hwang, W.S. A Gallium Oxide-Graphene Oxide Hybrid Composite for Enhanced Photocatalytic Reaction. Nanomaterials 2016, 6, 127. https://doi.org/10.3390/nano6070127
Kim S, Han KI, Lee IG, Park WK, Yoon Y, Yoo CS, Yang WS, Hwang WS. A Gallium Oxide-Graphene Oxide Hybrid Composite for Enhanced Photocatalytic Reaction. Nanomaterials. 2016; 6(7):127. https://doi.org/10.3390/nano6070127
Chicago/Turabian StyleKim, Seungdu, Kook In Han, In Gyu Lee, Won Kyu Park, Yeojoon Yoon, Chan Sei Yoo, Woo Seok Yang, and Wan Sik Hwang. 2016. "A Gallium Oxide-Graphene Oxide Hybrid Composite for Enhanced Photocatalytic Reaction" Nanomaterials 6, no. 7: 127. https://doi.org/10.3390/nano6070127
APA StyleKim, S., Han, K. I., Lee, I. G., Park, W. K., Yoon, Y., Yoo, C. S., Yang, W. S., & Hwang, W. S. (2016). A Gallium Oxide-Graphene Oxide Hybrid Composite for Enhanced Photocatalytic Reaction. Nanomaterials, 6(7), 127. https://doi.org/10.3390/nano6070127