Sol-Gel-Synthesis of Nanoscopic Complex Metal Fluorides
Abstract
:1. Introduction
2. Results and Discussion
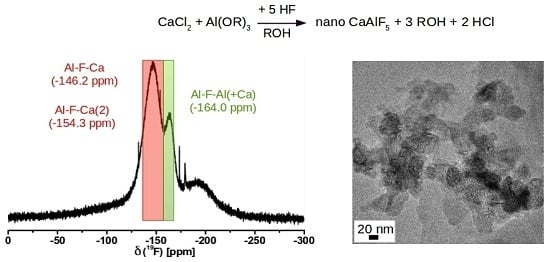
2.1. CaAlF5
2.2. Ca2AlF7
2.3. CaAl2F8
2.4. LiMgAlF6
3. Materials and Methods
3.1. Synthesis of Complex Metal Fluoride Sols
3.2. Analytical Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Du, M.H. Chemical stability and Ce doping of LiMgAlF6 neutron scintillator. J. Alloys Compd. 2015, 622, 925–928. [Google Scholar] [CrossRef]
- Belsare, P.D.; Joshi, C.P.; Moharil, S.V.; Omanwarc, S.K.; Muthald, P.L.; Dhopted, S.M. Preparation and characterization of LiAEAlF6 Eu (AE = Mg, Ca, Sr or Ba) phosphors. J. Lumin. 2009, 129, 135–139. [Google Scholar] [CrossRef]
- Samtleben, T.A.; Hulliger, E. LiCaAlF6 and LiSrAlF6: Tunable solid state laser host materials. Opt. Lasers Eng. 2005, 43, 251–262. [Google Scholar] [CrossRef]
- Van der Kolk, E.; Dorenbos, P.; van Eijk, C.W.E. Luminescence excitation study of the higher energy states of Pr3+ and Mn2+ in SrAlF5, CaAlF5, and NaMgF3. J. Appl. Phys. 2004, 95, 7867–7872. [Google Scholar] [CrossRef]
- Gektin, A.; Shiran, N.; Neicheva, S.; Gavrilyuk, V.; Bensalah, A.; Fukuda, T.; Shimamura, K. LiCaAlF6:Ce crystal: A new scintillator. Nucl. Instrum. Methods Phys. Res. Sect. A 2002, 486, 274–277. [Google Scholar] [CrossRef]
- Tao, F.; Hong, G.Y.; Zhu, S.F.; You, H.; Zhou, X.; Zhao, B. Synthesis and spectroscopic characteristics of LiMgAlF6: RE3+ (RE = Eu, Tm, Gd). Phys. Status Solidi A-Appl. Res. 1998, 165, 303–308. [Google Scholar] [CrossRef]
- Tao, F.; Zhou, X.J.; Zhu, S.F.; Zhao, B.; Hong, G.; You, H. Synthesis and luminescence characteristics of LiMgAlF6:Ln3+ (Ln = Ce, Eu, Tb). Cryst. Res. Technol. 1997, 32, 849–855. [Google Scholar] [CrossRef]
- Bessoi, M.; Soren, S.; Parhi, P. Rapid microwave mediated hydrothermal synthesis of complex ternary fluorides. Ceram. Int. 2016, 42, 3697–3700. [Google Scholar] [CrossRef]
- Zakalyukin, R.M.; Fedorov, P.P. Classification of fluoroaluminate glasses. Inorg. Mater. 2003, 39, 640–644. [Google Scholar] [CrossRef]
- Ehrt, D.; Vogel, W. Fluoroaluminate glass. J. Fluorine Chem. 1985, 29, 54. [Google Scholar] [CrossRef]
- Krauß, M.; Ehrt, D.; Heide, K.; Vogel, W. Phasenanalytische Untersuchungen im System CaF2-AlF3. Z. Chem. 1984, 24, 247–250. [Google Scholar] [CrossRef]
- Ehrt, D.; Krauß, M.; Erdmann, C.; Vogel, W. Fluoroaluminatgläser;1) Systeme CaF2-AlF3 und MgF2-CaF2-AlF3. Z. Chem. 1982, 22, 315–316. [Google Scholar] [CrossRef]
- Zhang, X.; Quan, Z.; Yang, J.; Yang, P.; Lian, H.; Lin, J. Solvothermal synthesis of well-dispersed NaMgF3 nanocrystals and their optical properties. J. Colloid Interface Sci. 2009, 329, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Kemnitz, E.; Gross, U.; Rudiger, S.; Shekar, S.H. Amorphous Metal Fluorides with Extraordinary High Surface Areas. Angew. Chem. Int. Ed. 2003, 42, 4251–4254. [Google Scholar] [CrossRef] [PubMed]
- Gross, U.; Ruediger, S.; Kemnitz, E. Alkaline earth fluorides and their complexes: A sol-gel fluorination study. Solid State Sci. 2007, 9, 838–842. [Google Scholar] [CrossRef]
- Ahrens, M.; Scholz, G.; Feist, M.; Kemnitz, E. Application of an alkoxide sol-gel route for the preparation of complex fluorides of the MAlF4 (M = K, Cs), M3AlF6 (M = Li, Na, K), and Na5Al3F14 type. Solid State Sci. 2006, 8, 798–806. [Google Scholar] [CrossRef]
- Kohl, J.; Wiedemann, D.; Nakhal, S.; Bottke, P.; Ferro, N.; Bredow, T.; Kemnitz, E.; Wilkening, M.; Heitjanse, P.; Lerch, M. Synthesis of ternary transition metal fluorides Li3MF6 via a sol-gel route as candidates for cathode materials in lithium-ion batteries. J. Mater. Chem. 2012, 22, 15819–15827. [Google Scholar] [CrossRef]
- Krahl, T.; Scheurell, K.; Kemnitz, E. Novel aspects in the chemistry of the non-aqueous fluorolytic sol-gel synthesis of nanoscaled homodisperse MgF2 sols for antireflective coatings. J. Mater. Chem. C 2016, 4, 1454–1466. [Google Scholar] [CrossRef]
- Ruediger, S.K.; Gross, U.; Feist, M.; Prescott, H.A.; Shekar, S.C.; Troyanova, S.I.; Kemnitz, E. Non-aqueous synthesis of high surface area aluminium fluoride—A mechanistic investigation. J. Mater. Chem. 2005, 15, 588–597. [Google Scholar] [CrossRef]
- Scheurell, K.; Kemnitz, E.; Garcia-Juan, P.; Eicher, J.; Lintner, B.; Hegmann, J.; Jahn, R.; Hofmann, T.; Löbmann, P. Porous MgF2 antireflective λ/4 films prepared by sol-gel processing: Comparison of synthesis approaches. J. Sol-Gel Sci. Technol. 2015, 76, 82–89. [Google Scholar] [CrossRef]
- Noack, J.; Scheurell, K.; Kemnitz, E.; Garcia-Juan, P.; Rau, H.; Lacroix, M.; Eicher, J.; Lintner, B.; Sontheimer, T.; Hofmann, T.; et al. MgF2 antireflective coatings by sol-gel processing: Film preparation and thermal densification. J. Mater. Chem. 2012, 22, 18535–18541. [Google Scholar] [CrossRef]
- Rehmer, A.; Scheurell, K.; Kemnitz, E. Formation of nanoscopic CaF2 via a fluorolytic sol-gel process for antireflective coatings. J. Mater. Chem. C 2015, 3, 1716–1723. [Google Scholar] [CrossRef]
- Kemnitz, E.; Noack, J. The non-aqueous fluorolytic sol-gel synthesis of nanoscaled metal fluorides. Dalton Trans. 2015, 44, 19411–19431. [Google Scholar] [CrossRef] [PubMed]
- Carta, D.; Pickup, D.M.; Knowles, J.C.; Smith, M.E.; Newport, R.J. Sol-gel synthesis of the P2O5-CaO-Na2O-SiO2 system as a novel bioresorbable glass. J. Mater. Chem. 2005, 15, 2134–2140. [Google Scholar] [CrossRef]
- Scholz, G.; Stosiek, C.; Noack, J.; Kemnitz, E. Local fluorine environments in nanoscopic magnesium hydr(oxide) fluorides studied by 19F MAS NMR. J. Fluorine Chem. 2011, 132, 1079–1085. [Google Scholar] [CrossRef]
- Craig, D.F.; Brown, J.J. Phase equilibria in system CaF2-AlF3. J. Am. Ceram. Soc. 1977, 60, 396–398. [Google Scholar] [CrossRef]
- Millet, J.P.; Rolin, M. Study of the median part of the phase CaF2-AlF3—Confirmation of a stability field for the compound Ca2AlF7. Rev. Int. Hautes Temp. Refract. 1981, 18, 287–292. [Google Scholar]
- Fedotiev, P.P.; Ilyinskii, V. Fusibility of the ternary system NaF-CaF2-AIF3. Z. Anorg. Allgem. Chem. 1923, 129, 93–107. [Google Scholar]
- Holm, J.L. Phase equilibria in system CaF2-AlF3. Acta Chem. Scand. 1965, 19, 1512–1514. [Google Scholar] [CrossRef]
- Hemon, A.; Courbion, G. Refinement of the room temperature structure of α-CaAlF5. Acta Crystallogr. Sect. C-Cryst. Struct. Commun. 1991, 47, 1302–1303. [Google Scholar] [CrossRef]
- Body, M.; Silly, G.; Legein, C.; Buzaréa, J.-Y.; Calvayraca, F.; Blahad, P. Structural investigations of beta-CaAlF5 by coupling powder XRD, NMR, EPR and spectroscopic parameter calculations. J. Solid State Chem. 2005, 178, 3655–3661. [Google Scholar] [CrossRef]
- Body, M.; Silly, G.; Legein, C.; Buzare, J.Y. Correlation between 19F environment and isotropic chemical shift in barium and calcium fluoroaluminates. Inorg. Chem. 2004, 43, 2474–2485. [Google Scholar] [CrossRef] [PubMed]
- Domesle, R.; Hoppe, R. The crystal structure of Ca2AlF7. Z. Kristall. 1980, 153, 317–328. [Google Scholar]
- Kiczenski, T.J.; Du, L.-S.; Stebbins, J.F. F-19 NMR study of the ordering of high field strength cations at fluoride sites in silicate and aluminosilicate glasses. J. Non-Cryst. Solids 2004, 337, 142–149. [Google Scholar] [CrossRef]
- Kiczenski, T.J.; Stebbins, J.F. Fluorine sites in calcium and barium oxyfluorides: F-19 NMR on crystalline model compounds and glasses. J. Non-Cryst. Solids 2002, 306, 160–168. [Google Scholar] [CrossRef]
- Balic-Zunic, T.; Garavelli, A.; Mitolo, D.; Acquafredda, P.; Leonardsen, E. Jakobssonite, CaAlF5, a new mineral from fumaroles at the Eldfell and Hekla volcanoes, Iceland. Mineral. Mag. 2012, 76, 751–760. [Google Scholar] [CrossRef]
- Kampf, A.R.; Colombo, F.; del Tanago, J.G. Carlhintzeite, Ca2AlF7 center dot H2O, from the Gigante granitic pegmatite, Cordoba province, Argentina: Description and crystal structure. Mineral. Mag. 2010, 74, 623–632. [Google Scholar] [CrossRef]
- Karg, M.; Scholz, G.; Koenig, R.; Kemnitz, E. Mechanistic insight into formation and changes of nanoparticles in MgF2 sols evidenced by liquid and solid state NMR. Dalton Trans. 2012, 41, 2360–2366. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.L.; Holm, B.J. Phase relations and thermodynamic properties in the ternary reciprocal system LiF-NaF-Na3AlF6-Li3AlF6. Thermochim. Acta 1973, 6, 375–398. [Google Scholar] [CrossRef]
- Voronkov, M.G.; Boyarkina, E.V.; Gebel, I.A.; Albanov, A.I.; Basenko, S.V. Cleavage of the C-Si bond in trifluoro(phenyl)silane with aliphatic alcohols. Russ. J. Gen. Chem. 2005, 75, 1927–1929. [Google Scholar] [CrossRef]
- JCPDS-ICDD, International Centre for Diffraction Data: PDF-2 Database; PCPDFWIN Version 2.2; ICDD: Newtown Square, PA, USA, 2001.













© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehmer, A.; Scheurell, K.; Scholz, G.; Kemnitz, E. Sol-Gel-Synthesis of Nanoscopic Complex Metal Fluorides. Nanomaterials 2017, 7, 362. https://doi.org/10.3390/nano7110362
Rehmer A, Scheurell K, Scholz G, Kemnitz E. Sol-Gel-Synthesis of Nanoscopic Complex Metal Fluorides. Nanomaterials. 2017; 7(11):362. https://doi.org/10.3390/nano7110362
Chicago/Turabian StyleRehmer, Alexander, Kerstin Scheurell, Gudrun Scholz, and Erhard Kemnitz. 2017. "Sol-Gel-Synthesis of Nanoscopic Complex Metal Fluorides" Nanomaterials 7, no. 11: 362. https://doi.org/10.3390/nano7110362
APA StyleRehmer, A., Scheurell, K., Scholz, G., & Kemnitz, E. (2017). Sol-Gel-Synthesis of Nanoscopic Complex Metal Fluorides. Nanomaterials, 7(11), 362. https://doi.org/10.3390/nano7110362