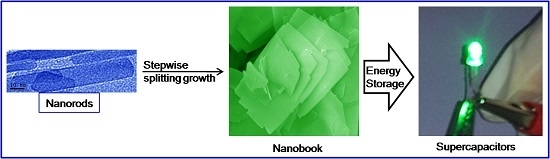
Stepwise Splitting Growth and Pseudocapacitive Properties of Hierarchical Three-Dimensional Co3O4 Nanobooks
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Characterization
2.2. Synthesis of Co3O4 Nanobooks
2.3. Electrochemical Measurements
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhou, X.; Shen, X.; Xia, Z.; Zhang, Z.; Li, J.; Ma, Y.; Qu, Y.; Cen, Q. Hollow fluffy Co3O4 cages as efficient electro-active materials for supercapacitors and oxygen evolution reaction. ACS Appl. Mater. Interfaces 2015, 7, 20322–20331. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, L.; Tan, X.; Holt, C.M.B.; Zahiri, B.; Olsen, B.C.; Mitlin, D. Supercapacitive properties of hydrothermally synthesized Co3O4 nanostructures. J. Phys. Chem. C 2011, 115, 17599–17605. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Wang, T. Morphology-controllable synthesis of cobalt oxalates and their conversion to mesoporous Co3O4 nanostructures for application in supercapacitors. Inorg. Chem. 2011, 50, 6482–6492. [Google Scholar] [CrossRef] [PubMed]
- Xuan, L.; Chen, L.; Yang, Q.; Chen, W.; Hou, X.; Jiang, Y.; Zhang, Q.; Yuan, Y. Engineering 2D multi-layer graphene-like Co3O4 thin sheets with vertically aligned nanosheets as basic building units for advanced pseudocapacitor materials. J. Mater. Chem. A 2015, 3, 17525–17533. [Google Scholar] [CrossRef]
- Yuan, C.; Yang, L.; Hou, L.; Shen, L.; Zhang, X.; Lou, X.W. Growth of ultrathin mesoporous Co3O4 nanosheetarrays on Ni foam for high-performance electrochemical capacitors. Energy Environ. Sci. 2012, 5, 7883–7887. [Google Scholar] [CrossRef]
- Hu, H.; Guan, B.; Xia, B.; Lou, X.W. Designed formation of Co3O4/NiCo2O4 double-shelled nanocages with enhanced pseudocapacitive and electrocatalytic properties. J. Am. Chem. Soc. 2015, 137, 5590–5595. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhu, T.; Wu, H.B.; Xu, R.; Chen, J.S.; Lou, X.W. Porous Co3O4 nanowires derived from long Co(CO3)0.5(OH)·0.11H2O nanowires with improved supercapacitive properties. Nanoscale 2012, 4, 2145–2149. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Chen, J.S.; Lou, X.W. Shape-controlled synthesis of porous Co3O4 nanostructures for application in supercapacitors. J. Mater. Chem. 2010, 20, 7015–7020. [Google Scholar] [CrossRef]
- Zhang, F.; Yuan, C.; Zhu, J.; Wang, J.; Zhang, X.; Lou, X.W. Flexible films derived from electrospun carbon nanofibers incorporated with Co3O4 hollow nanoparticles as self-supported electrodes for electrochemical capacitors. Adv. Funct. Mater. 2013, 23, 3909–3915. [Google Scholar] [CrossRef]
- Ning, F.; Shao, M.; Zhang, C.; Xu, S.; Wei, M.; Duan, X. Co3O4@layered double hydroxide core/shell hierarchical nanowire arrays for enhanced supercapacitance performance. Nano Energy 2014, 7, 134–142. [Google Scholar] [CrossRef]
- Deori, K.; Ujjain, S.K.; Sharma, R.K.; Deka, S. Morphology controlled synthesis of nanoporous Co3O4 nanostructures and their charge storage characteristics in supercapacitors. ACS Appl. Mater. Interfaces 2013, 5, 10665–10672. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.-H.; Tu, J.-P.; Wang, X.-L.; Gu, C.-D.; Zhao, X.-B. Mesoporous Co3O4 monolayer hollow-sphere array as electrochemical pseudocapacitor material. Chem. Commun. 2011, 47, 5786–5788. [Google Scholar] [CrossRef] [PubMed]
- Rakhi, R.B.; Chen, W.; Cha, D.; Alshareef, H.N. Substrate dependent self-organization of mesoporous cobalt Oxide nanowires with remarkable pseudocapacitance. Nano Lett. 2012, 12, 2559–2567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Z.; Wang, Y.; Xie, Y.-L.; Cheng, T.; Lai, W.-Y.; Pang, H.; Huang, W. Porous hollow Co3O4 with rhombic dodecahedral structures for high-performance supercapacitors. Nanoscale 2014, 6, 14354–14359. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Pan, A.; Zhu, Q.; Nie, Z.; Zhang, Y.; Tang, Y.; Liang, S.; Cao, G. Facile synthesis of nanorod-assembled multi-shelled Co3O4 hollow microspheres for high-performance supercapacitors. J. Power Sources 2014, 272, 107–112. [Google Scholar] [CrossRef]
- Meng, F.; Fang, Z.; Li, Z.; Xu, W.; Wang, M.; Liu, Y.; Zhang, J.; Wang, W.; Zhao, D.; Guo, X. Porous Co3O4 materials prepared by solid-state thermolysis of a novel Co-MOF crystal and their superior energy storage performances for supercapacitors. J. Mater. Chem. A 2013, 1, 7235–7241. [Google Scholar] [CrossRef]
- Kung, C.-W.; Chen, H.-W.; Lin, C.-Y.; Vittal, R.; Ho, K.-C. Synthesis of Co3O4 nanosheets via electrodeposition followed by ozone treatment and their application to high-performance supercapacitors. J. Power Sources 2012, 214, 91–99. [Google Scholar] [CrossRef]
- Jena, A.; Munichandraiah, N.; Shivashankar, S.A. Morphology controlled growth of meso-porous Co3O4 nanostructures and study of their electrochemical capacitive behavior. J. Electrochem. Soc. 2012, 159, A1682–A1689. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, T.; Yu, X.; Zhang, H.; Guan, H.; Lu, B. Nanoforest of hierarchical Co3O4@NiCo2O4 nanowire arrays for high-performance supercapacitors. Nano Energy 2013, 2, 586–594. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Cheng, C.; Li, H.; Zhang, J.; Gong, H.; Fan, H.J. Co3O4 nanowire@MnO2 ultrathin nanosheet core/shell arrays: A new class of high-performance pseudocapacitive materials. Adv. Mater. 2011, 23, 2076–2081. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Yuan, C.; Zhang, M.; Xi, B.; Qian, Y. Controllable synthesis of mesoporous Co3O4 nanostructures with tunable morphology for application in supercapacitors. Chem. Eur. J. 2009, 15, 5320–5326. [Google Scholar] [CrossRef] [PubMed]
- Razmjoo, P.; Sabour, B.; Dalvand, S.; Aghazadeh, M.; Ganjali, M.R. Porous Co3O4 nanoplates: Electrochemical synthesis, characterization and investigation of supercapacitive performance. J. Electrochem. Soc. 2014, 161, D293–D300. [Google Scholar] [CrossRef]
- Fan, Y.; Shao, G.; Ma, Z.; Wang, G.; Shao, H.; Yan, S. Ultrathin nanoflakes assembled 3D hierarchical mesoporous Co3O4 nanoparticles for high-rate pseudocapacitors. Part. Part. Syst. Charact. 2014, 31, 1079–1083. [Google Scholar] [CrossRef]
- Yang, W.; Gao, Z.; Ma, J.; Wang, J.; Wang, B.; Liu, L. Effects of solvent on the morphology of nanostructured Co3O4 and its application for high-performance supercapacitors. Electrochim. Acta 2013, 112, 378–385. [Google Scholar] [CrossRef]
- Yuan, C.; Yang, L.; Hou, L.; Shen, L.; Zhang, F.; Li, D.; Zhang, X. Large-scale Co3O4 nanoparticles growing on nickel sheets via a one-step strategy and their ultra-highly reversible redox reaction toward supercapacitors. J. Mater. Chem. 2011, 21, 18183–18185. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, S.; Fang, S.; Jia, D.; Su, H.; Zhou, W.; Wiley, J.B.; Li, F. Plum-like and octahedral Co3O4 single crystals on and around carbon nanotubes: Large scale synthesis and formation mechanism. RSC Adv. 2012, 2, 3496–3501. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, A.; Liu, S.; Zhao, J.; Fang, S.; Jia, D.; Li, F. Free-standing and porous hierarchical nanoarchitectures constructed with cobalt cobaltite nanowalls for supercapacitors with high specific capacitances. J. Power Sources 2012, 219, 140–146. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, S.; Li, F.; Zhang, A.; Zhao, J.; Fang, S.; Jia, D. 3D hierarchical Co3O4 twin-spheres with an urchin-like structure: Large-Scale synthesis, multistep-splitting growth and electrochemical pseudocapacitors. Adv. Funct. Mater. 2012, 22, 4052–4059. [Google Scholar] [CrossRef]
- Gong, Y.; Gong, F.; Wang, C.; Zheng, H.; Li, F. Porous and single crystalline Co3O4 nanospheres for pseudocapacitors with enhanced performance. RSC Adv. 2015, 5, 27266–27272. [Google Scholar] [CrossRef]
- Wang, X.; Ding, J.; Yao, S.; Wu, X.; Feng, Q.; Wang, Z.; Geng, B. High supercapacitor and adsorption behaviors of flower-like MoS2 nanostructures. J. Mater. Chem. A 2014, 2, 15958–15963. [Google Scholar] [CrossRef]
- Xin, Z.; Yan, X.; Sun, Y.; Yu, Y.; Zhang, G.; Shen, Y.; Liang, Q.; Liao, Q.; Yue, Z. Temperature-dependent electrochemical capacitive performance of the α-Fe2O3 hollow nanoshuttles as supercapacitor electrodes. J. Colloid Interface Sci. 2016, 466, 291–296. [Google Scholar]
- Pujari, R.B.; Lokhande, A.C.; Kim, J.H.; Lokhande, C.D. Bath temperature controlled phase stability of hierarchical nanoflakes CoS2 thin films for supercapacitor application. RSC Adv. 2016, 6, 40593–40601. [Google Scholar] [CrossRef]
- Guan, B.Y.; Yu, L.; Wang, X.; Song, S.; Lou, X.W. Formation of onion-like NiCo2S4 particles via sequential ion-exchange for hybrid supercapacitors. Adv. Mater. 2017, 29, 1605051. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, L.; Zhang, D.; Chen, S.; Coxon, P.R.; He, X.; Coxon, P.R.; He, X.; Coto, M.; Kim, H.K. A universal synthetic route to carbon nanotube/transition metal oxide nano-composites for lithium ion batteries and electrochemical capacitors. Sci. Rep. 2016, 6, 37752. [Google Scholar] [CrossRef] [PubMed]










© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Lu, S.; Gong, F.; Liu, H.; Li, F. Stepwise Splitting Growth and Pseudocapacitive Properties of Hierarchical Three-Dimensional Co3O4 Nanobooks. Nanomaterials 2017, 7, 81. https://doi.org/10.3390/nano7040081
Chen H, Lu S, Gong F, Liu H, Li F. Stepwise Splitting Growth and Pseudocapacitive Properties of Hierarchical Three-Dimensional Co3O4 Nanobooks. Nanomaterials. 2017; 7(4):81. https://doi.org/10.3390/nano7040081
Chicago/Turabian StyleChen, Huilong, Shuang Lu, Feilong Gong, Huanzhen Liu, and Feng Li. 2017. "Stepwise Splitting Growth and Pseudocapacitive Properties of Hierarchical Three-Dimensional Co3O4 Nanobooks" Nanomaterials 7, no. 4: 81. https://doi.org/10.3390/nano7040081
APA StyleChen, H., Lu, S., Gong, F., Liu, H., & Li, F. (2017). Stepwise Splitting Growth and Pseudocapacitive Properties of Hierarchical Three-Dimensional Co3O4 Nanobooks. Nanomaterials, 7(4), 81. https://doi.org/10.3390/nano7040081