1. Introduction
Study of effective photocatalysts lies in following conditions: (i) the prepared photocatalysts are capable of harvesting the solar energy of full wavelength as much as possible; (ii) high photocatalysis efficiency [
1,
2]. Due to the odorless, non-toxic, and chemically stable properties of TiO
2, it is widely employed and deeply analyzed for various kinds of applications [
3,
4,
5,
6,
7,
8,
9,
10]. However, TiO
2-based photocatalysts are only able to capture the ultraviolet (UV) part of the solar light; the UV light energy is comprised of only 4% of the total solar energy reaching earth, along with a rather low photocatalysis efficiency being lower than 1%, in contrast to 43% when visible light region is concerned [
11,
12]. As a consequence, the current focus is placed on exploration of new visible light-active photocatalysts or modification of existing photocatalysts to extend the solar light absorption to the visible light region [
13].
As one of most widespread applications of TiO
2 materials, dye-sensitized solar cells (DSSC), involves the use of a nano-porous TiO
2 film as semiconducting electrodes, with high specific surface area [
14]. The DSSC is a photoelectrochemical solar cell, which employs organic compounds containing metallic Ru, Os, etc. as dye sensitizers and selects proper redox electrolytes. The photoelectrical efficiency of a DSSC was recently reported to achieve as higher than 11%, which can also arrive at 6% as far as large-scale applications are concerned [
15,
16,
17,
18]. Such efficiencies are much higher than that for un-sensitized photocatalysts. However, most organic dyes used in DSSC are unstable, causing instability of the DSSC [
16]. To address this, stable narrow-band-gap semiconductors can be resorted and used as a sensitizer for visible light-activation of TiO
2, replacing the organic dye in DSSC. Similar to the function obtained in DSSC, the semiconductor with a narrow band gap can efficiently harvest the solar energy, while wide-band-gap TiO
2 is able to separate the photo-generated charge carriers.
The significance of using visible light-active photocatalysts to sensitize TiO
2 is to improve solar light absorption of TiO
2 [
19]. Different energy band gaps between the sensitizer and TiO
2 lead to the generation of a built-in electric field [
20], causing the photo-excited charge carriers to inject from one semiconductor to the other [
21]. This inhibits the recombination of the generated charge carriers, in the meantime, the photo-generated electrons and holes can be well separated, improving the photocatalytic activity [
22]. As a result, such combined semiconductors always exhibit a higher photocatalytic activity than that of the single-component semiconductor [
23]. In order to combine semiconductors with different energy band gaps, we need to consider (i) band gaps of these semiconductors; (ii) positions of the valence band (VB) and conduction band (CB); and (iii) the match of crystalline forms of these semiconductors. The methods and procedures used to prepare the combined semiconductors also have an influence on the final photocatalytic activity [
24].
For the first time, this paper reports the sensitization of TiO2 nanotubes by coupling a visible light-active semiconductor Bi2O3, affording a good a visible light-absorptivity; this thus also indicates the enhancement of the solar light absorption. Selecting Bi2O3 for sensitization of TiO2 is due to (i) its narrow direct band gap of 2.8 eV; and (ii) suitable VB and CB positions for transporting and separating the photo-generated charge carriers. Because the photo-generated electrons and holes can be trapped and transported by these different semiconductors, both the photo-generated electrons and holes can be concentrated simultaneously, enhancing the oxidation/reduction capability of the electrodes in prepared solar cells. The photocatalytic and photoelectric conversion efficiencies are improved correspondingly. This paper presents the detailed synthesis of the visible light-active composite of Bi2O3-sensitized TiO2 nanotube film. The structure and photoelectrical properties of the composite are also well unraveled.
2. Experimental
2.1. Materials
Bismuth nitrate hydrate (Bi(NO3)2·5H2O) of AR grade was supplied by Guangzhou Chemical Company (Guangzhou, China). Tetra-n-butyl titanate (Ti(OC4H9)4) of AR grade was obtained from Xinhua Active Materials Institute (Changzhou, Jiangsu, China). Diethanol amine (NH(C2H5OH)2 ) of AR grade was purchased from Luoyang Chemical Reagent Factory (Luoyang, China). Polyethylene glycol (MW 20,000) was provided by UNI-CHEM (Hong Kong, China), with AR purity. All other chemicals were of AR grade and used as received.
2.2. Synthesis of TiO2 Nanotubes
The synthesis of TiO2 nanotubes was based on a hydrothermal route. Specifically, 2.0 g of purchased TiO2 with anatase form was mixed into a NaOH solution (10 mol/L) by continuously stirring at 110 °C for 12 h, followed by cooling to room temperature. The white precipitate was then collected and thoroughly washed with deionized (DI) water until the electrical conductivity of the supernatant was lower than 70 μS/cm. The washing procedures were continued by dipping the precipitate into a HCl solution (0.1 mol/L) and letting it stand for 5 h, and then by further washing with DI water until the electrical conductivity of the supernatant was lower than 5 μS/cm. Afterwards, the washed TiO2 was vacuum-dried at 60 °C for 72 h.
2.3. Preparation of TiO2 Sol-Based Film
Using Ti(OC4H9)4 as a raw material and NH(C2H5OH)2 as an inhibitor to impede fast hydrolyzation of the titanate. The addition ratio of the Ti(OC4H9)4, EtOH, DI water and NH(C2H5OH)2 was kept at 1:26.6:1:1. A precise amount of Ti(OC4H9)4 was dissolved into ethyl alcohol (EtOH), along with addition of NH(C2H5OH)2. The mixture was agitated for 1 h, leading to liquid A. The DI water was homogenized with the remaining EtOH, forming liquid B which was then stepwise added into the liquid A through a separating funnel, followed by continuous stirring for 0.5 h and then letting it stand for 24 h. The resulting stable TiO2 sol was finally obtained. A fluorine-doped tin oxide (FTO) glass was then used as a carrier after being thoroughly washed by sonication treatment and subsequently dried. Two layers of TiO2 sol-based film was then deposited onto the FTO glass with a self-made film stretching machine at a stretching speed of 2 mm/s. Afterwards, the TiO2 sol-based film was thermally treated in a muffle furnace being heated to 500 °C at a heating rate of 2 °C/min, and kept at 500 °C for 1 h, followed by cooling to room temperature.
2.4. Preparation of TiO2 Nanotube-Based Film Electrodes by a Powder-Coating Method
TiO2 nanotubes (3.0 g) were placed in a mortar, followed by addition of 1 mL EtOH solution containing 10% diacetone and then ball-milling for 30 min. Afterwards, a mixture of DI water (4.5 mL), Triton X-100 (0.1 mL) and 30% (in weight, 0.9 g) of polyethylene glycol (MW 20,000) was added, and then ball-milled for 30 min. The as-prepared mixed dispersion with a proper concentration was applied onto the above-prepared conducting FTO glass with two layers of TiO2 film by a powder-coating method. A scotch tape (with around 40 μm thickness) was attached vertically onto the two sides of the conducting FTO glass coated with the stretched film, and then the prepared TiO2 slurry was applied onto the conducting glass by rolling a glass bar. Rolling once in the same direction could result in the best film forming effect. The formed film with a size of 1 cm × 1 cm was vacuum-dried at 60 °C for 12 h, and then put into a muffle furnace, heated to 400 °C (at a heating rate of 2 °C/min), kept at that temperature for 1 h, and finally cooled to 80 °C for storage.
2.5. Sensitization of TiO2 Nanotubes with Bi2O3
A precursor solution of Bi
2O
3 was first prepared as follows. Bi(NO
3)
2 solutions were prepared with molar concentrations of 0.01, 0.05, 0.15, 0.25, and 0.5 mol/L. The as-obtained TiO
2 nanotube film was dipped into the Bi(NO
3)
2 solution and allowed to stand for 24 h, followed by washing with DI water and then drying under vacuum at 50 °C for 12 h. Afterwards, the treated film was placed into a muffle furnace which was heated to 400 °C at a heating rate of 2 °C/min, kept at that temperature for 5 h, and finally cooled naturally to 80 °C for storage. A series of samples were prepared, including pure TiO
2 nanotube-based film (TNT), 0.01BiTNT, 0.05BiTNT, 0.15BiTNT, 0.25BiTNT, and 0.5BiTNT, where, for example, 0.01BiTNT represents that the product was prepared with the initial Bi(NO
3)
2 (a Bi
2O
3 precursor) solution of 0.01 mol/L. For clear demonstration of the sensitization effect of Bi
2O
3 on the TiO
2 nanotubes, this study also investigated a simple mixture of TiO
2 nanotubes and Bi
2O
3 particles being prepared by calcination of Bi(NO
3)
2 according to the above thermal treatment procedures. The weight percentage of Bi
2O
3 particles as used to mix with the TiO
2 nanotubes was determined by quantification results of the energy-dispersive X-ray (EDX) spectrum for the optimized sample (
Figure 1).
2.6. Characterizations
Transmission electron microscopy (TEM) observation was conducted on a JEOL JEM-1010 system (Tokyo, Japan). A JEM-2010HR system was used for the high-resolution TEM (HRTEM) analysis. Backscattered-electron (BSE) and EDX mapping images were captured on a tabletop microscope (TM3030Plus, Hitachi, Japan) being equipped with an EDX spectrometer (Quantax 70, Bruker, Germany). X-ray diffraction (XRD) analysis proceeded on a powder diffractometer (X’Pert-PRO, PANalytical, Philips, Amsterdam, The Netherlands); the test conditions were set as: Cu-Kα radiation, working voltage of 40 KV, working current of 40 mA, and scanning speed of 1.5°/min. The UV/Vis diffuse reflectance spectra (UV/Vis DRS) were captured from a UV-Vis-NIR spectrophotometer (LAMBDA 750, PerkinElmer, Norwalk, CT, USA), equipped with an integrating sphere. X-ray photoelectron spectroscopy (XPS) spectra were obtained from XSAM800-XPS equipment (Kratos, UK) with a Ma-Kα X ray source.
2.7. Photoelectric Conversion Properties of Bi2O3 Sensitized TiO2 Nanotube film
The dye-sensitized TiO
2 nanotube-based anode film was replaced by Bi
2O
3-sensitized TiO
2 nanotube film. Pt was then thermally deposited onto the conducting FTO glass as the other electrode. The two electrodes are assembled into a solar cell with a sandwiched structure, as displayed in
Figure 2. Under xenon lamp irradiation conditions (300 W, AM 1.5, 100 mW/cm
2, with the wavelengths below 400 nm filtered), IV curves are measured for the solar cell using electrochemical workstation. The incident-photon-to-current efficiency (IPCE) measurements were conducted using electrochemical workstation Zahner (PP211, CIMPS-pcs, Kronach, Germany) at the visible wavelengths of 400, 430, 450, 475, 500, 550, and 600 nm under xenon lamp irradiation conditions (300 W, AM 1.5, 100 mW/cm
2).
A thermal deposition method was employed to fabricate the Pt counter electrode. A chloroplatinic acid EtOH solution with a proper concentration was first prepared at room temperature; specifically, 0.9 g of chloroplatinic acid was dissolved into 250 mL of EtOH. A dip-pulling method was then used to make a two-layer film onto a cleaned conductive glass with a self-made film-stretching machine. The thermal deposition was conducted at 390 °C (at a heating rate of 2 °C/min) and kept at the temperature for 0.5 h, followed by natural cooling.
The prepared electrolyte was an acetonitrile solution containing 0.1mol/L LiI, 0.05mol/L I2, and 0.5 mol/L Tert butyl pyridine.
3. Results and Discussion
This study first compares the microstructures of the typical sample, 0.05BiTNT, and a simple mixture of Bi
2O
3 and TiO
2 nanotubes, with the same weight percentage of Bi
2O
3 as estimated by the quantification result of the EDX spectrum for 0.05BiTNT (
Figure 1). From the EDX mapping images (
Figure 3), considerably better distribution of element Bi can be found for 0.05BiTNT (bottom row) as compared to the simple mixture (top row) with the Bi
2O
3 particles showing apparently unsatisfactory adhesion interactions with the TiO
2 matrix, which implies the importance and significance of the present sensitization method.
To further unravel the microstructure of the Bi
2O
3-sensitized TiO
2 nanotubes, TEM and HRTEM are presented in
Figure 4a–f. The TEM specimen was prepared by sampling from the coated FTO glass. Under low-magnification TEM (
Figure 4a,b), neat TiO
2 nanotubes show a large length-diameter ratio, with smooth tube walls. As shown in
Figure 4c,d, the Bi
2O
3-sensitized TiO
2 nanotube walls become coarsened, with the well-observed Bi
2O
3 nanoparticles coated on the walls. Under HRTEM (
Figure 4e,f), it can be noted that the formed Bi
2O
3 nanoparticles are not fully deposited uniformly on the TiO
2 nanotube walls, with a little amount of aggregates of Bi
2O
3 nanoparticles (highlighted by broken cycles). The average diameter of Bi
2O
3 nanoparticles can be calculated as around 10 nm.
The XRD patterns of the as-prepared coated FTO glass are shown in
Figure 5. The characteristic peak assignable to FTO glass substrate can be noted. The anatase TiO
2 crystal can also be well identified through the standard PDF cards of anatase (No. 89-4921) and its refection planes (highlighted by red dotted lines). As the concentration of the Bi
2O
3 precursor increases, the Bi
2O
3-related XRD bands gradually appear—as marked by blue dotted lines for showing the characteristic reflection planes of Bi
2O
3 (PDF#74-2351). Note that a low content of Bi
2O
3 for the sensitized samples such as 0.05BiTNT and the simple mixture of Bi
2O
3 and TiO
2 causes the XRD peaks characteristic of Bi
2O
3 to be undetectable. From the UV/Vis spectra shown in
Figure 6, the red shift of the absorption wavelength occurs for all the Bi
2O
3 sensitized TiO
2 nanotube films. The higher the concentration of Bi
2O
3 precursor, the larger the red-shift extent, indicating the corresponding higher content of final formed Bi
2O
3 nanoparticles in the Bi
2O
3 sensitized TiO
2 sample. This also indicates the successful sensitization of TiO
2 by Bi
2O
3 [
25].
To determine the elemental composition and obtain the corresponding chemical state information of the Bi
2O
3-sensitized TiO
2 nanotube surface, XPS analysis is used to measure the typical sample TNT (as a control) and 0.05BiTNT.
Figure 7a shows the full XPS spectra, verifying the elements Ti, O, Bi, and C (the element C most likely stems from the contaminant [
26]) included in 0.05BiTNT. Due to the incorporation of Bi
2O
3, an additional signal from Bi 4f can be well observed in the full XPS spectrum for 0.05BiTNT, in contrast to that for TNT. The high-resolution XPS spectrum of Bi 4f is given in
Figure 7b, and the two characteristic peaks at approximately 164.2 and 158.8 eV can be indexed to the Bi 4f
6/2 and Bi 4f
7/2 respectively [
27]. The binding energy of Bi as for 0.05BiTNT is the same as that for pure Bi
2O
3, indicating that the element Bi of 0.05BiTNT presents in the chemical state of Bi
2O
3. The binding energy of Bi for 0.05BiTNT is in great agreement with those for reported pure Bi
2O
3 [
28] and a Bi
2O
3–TiO
2 composite [
29], thus revealing that the element Bi of our 0.05BiTNT presents the chemical state of Bi
2O
3. This is also in good agreement with XRD analysis.
In the high-resolution XPS spectrum of Ti 2p (
Figure 7c), the Ti 2p energy level is split into two energy levels, namely Ti 2p
1/2 and Ti 2p
3/2 as a result of spin-orbital coupling of electrons. The binding energy positions of Ti 2p
1/2 and Ti 2p
3/2 can be found at approximately 464.0 and 458.3 eV respectively, being basically the same as that reported elsewhere [
30]. This also indicates that, both before and after the sensitization of TiO
2 with Bi
2O
3, the oxidation state of the element Ti keeps the same, i.e., Ti
4+, in the absence of Ti
3+, and thus reveals the high purity of TiO
2 [
31]. The broadening of the XPS peaks after the Bi
2O
3 incorporation (0.05BiTNT vs. TNT) can be attributed to the electron transfer interactions between the matrix TiO
2 and the sensitizer Bi
2O
3 [
32]. The high-resolution XPS spectra of O 1s are shown in
Figure 7d. Two peaks at 529.7 and 531.4 eV can be noticed by Gaussian fitting. The former can be indexed to the O of TiO
2; the latter most likely results from Bi
2O
3, which may also be correlated to OH species from chemical absorption or absorbed water [
25]. The lower binding energy of Bi–O is owing to the less negative changes of the covalent O of Bi
2O
3 as compared to that of the O of Ti–O [
33]. To conclude, it is believed that the TiO
2 nanotubes are effectively sensitized by Bi
2O
3 [
25].
Figure 8 shows the IV curves (obtained for the assembled solar cell with the Bi
2O
3 sensitized TiO
2 nanotube anode film). The corresponding PV curves of the Bi
2O
3 sensitized solar cell are presented in
Figure 9. The photoelectric conversion efficiency of the DSSC is not directly proportional to the amount of sensitizer Bi
2O
3, and there exists an optimized content of Bi
2O
3. Our optimal content of the sensitizer Bi
2O
3 corresponds to the initial Bi
2O
3 precursor concentration of 0.05 mol/L. The lower content of Bi
2O
3 is most likely to cause insufficient utilization of visible light, while an excess amount of Bi
2O
3 exerts an adverse effect on the light absorption property of the light anode material, revealing that an optimized content of Bi
2O
3 exists. In DSSC, the optimal absorbed dye concentration lies in the monolayer adsorption of dye molecules onto the porous TiO
2-based film electrode, which may be also applied to our case. To further prove the sensitization effect of Bi
2O
3 on TiO
2 nanotubes, a simple mixture of Bi
2O
3 particles and TiO
2 nanotubes is also investigated and compared with our optimized sample, 0.05BiTNT. The weight percentage of the Bi
2O
3 particles used for the mixing is estimated from the quantified result of the EDX spectrum for 0.05BiTNT (
Figure 1). The simple mixture-based solar cell presents much lower photoelectric conversion efficiency as compared to the sensitized counterpart (0.05BiTNT), thereby indicating the significance of the sensitization effect as a result of uniform distribution of Bi
2O
3 and interfacial adhesion between the Bi
2O
3 and TiO
2 nanotubes, as evidenced by EDX mapping images (
Figure 3), and TEM and HRTEM images (
Figure 4).
From
Table 1, it can be noted that, as the Bi
2O
3 content increases, the photoelectric conversion efficiency raises first and then exhibits a decreasing trend. The sample 0.05BiTNT presents the highest photoelectric conversion efficiency, followed by 0.15BiTNT > 0.5BiTNT > 0.25BiTNT > 0.01BiTNT > TNT. The un-sensitized TiO
2 nanotubes (namely TNT)-assembled DSSC possesses a rather unsatisfactory photovoltaic property; this is because the present visible light source cannot excites the TiO
2 nanotube film, with a wide energy band gap, to generate photocurrent and photovoltage, indicating the high significance of the present sensitization of TiO
2 nanotubes with Bi
2O
3. Furthermore, a IPCE spectrum of the typical sample, 0.05BiTNT, shows a gradual lowering of IPCE with increasing incident wavelengths (starting from 400 to 600 nm), as presented in
Figure 10.
4. Conclusions
In this study, a series of visible light-responsive Bi2O3 sensitized TiO2 nanotube anode films were prepared by combined hydrothermal reactions, dip-coating, and calcination. In terms of the photoelectric conversion efficiency of the assembled solar cells, the Bi2O3 precursor (Bi(NO3)2) concentration of 0.05 mol/L is estimated to be the optimal condition. The photoelectric conversion efficiency is not in direct proportion with the Bi2O3 content, and optimization needs to be performed. Similar to dye sensitization in DSSC, the monolayer adsorption of the sensitizer is most likely to be optimal for achieving high photoelectric conversion efficiency.
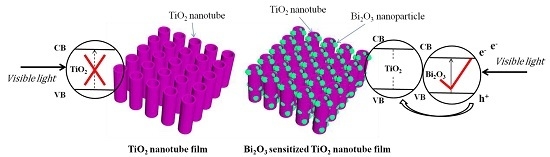
As a new-type visible light-active photocatalyst, Bi
2O
3 is popular in the field of semiconductor materials. Bi
2O
3 has an energy band gap of 2.8 eV (
ECB = 0.33 eV,
EVB = 3.13 eV) at room temperature [
34]. A single component photocatalyst, TiO
2 nanotubes, shows a rather unsatisfactory visible light-driven photocatalytic property, but after sensitization with Bi
2O
3, its photoelectric conversion efficiency is largely improved, as schematically shown in
Figure 11. Bi
2O
3 serves to absorb visible light energy to generate photoexcited electrons and holes, while TiO
2 works to collect and transport the generated charge carriers [
35]. Considering the different CB and VB positions of Bi
2O
3 and TiO
2, a built-in electric field can be generated as a result of the energy level difference between Bi
2O
3 and TiO
2, effectively separating the photo-generated electron-hole pairs and consequently improving the photocatalytic activity.