Calcination Method Synthesis of SnO2/g-C3N4 Composites for a High-Performance Ethanol Gas Sensing Application
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sample Characterization
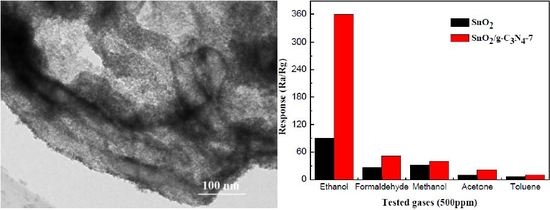
2.2. Gas-Sensing Performance
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of g-C3N4
3.3. Synthesis of the SnO2/g-C3N4Composite
3.4. Characterizations
3.5. Gas-Sensing Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hou, C.; Li, J.; Huo, D.; Luo, X.; Dong, J.; Yang, M.; Shi, X. A portable embedded toxic gas detection device based on a cross-responsive sensor array. Sens. Actuators B 2012, 161, 244–250. [Google Scholar] [CrossRef]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Ariyageadsakul, P.; Vchirawongkwin, V.; Kritayakornupong, C. Determination of toxic carbonyl species including acetone, formaldehyde, and phosgene by polyaniline emeraldine gas sensor using DFT calculation. Sens. Actuators B 2016, 232, 165–174. [Google Scholar] [CrossRef]
- Hui, G.H. Detection of Sulfur Hexafluoride Based on Stochastic Resonance and Miniaturized Sensor Array. Chin. J. Anal. Chem. 2010, 38, 984–988. [Google Scholar]
- Kaneti, Y.V.; Yue, J.; Moriceau, J.; Chen, C.; Liu, M.; Yuan, Y.; Jiang, X.; Yu, A. Experimental and theoretical studies on noble metal decorated tin oxide flower-like nanorods with high ethanol sensing performance. Sens. Actuators B 2015, 219, 83–93. [Google Scholar] [CrossRef]
- Kumar, N.; Srivastava, A.K.; Patel, H.S.; Gupta, B.K.; Varma, G.D. Facile Synthesis of ZnO–Reduced Graphene Oxide Nanocomposites for NO2 Gas Sensing Applications. Eur. J. Inorg. Chem. 2015, 2015, 1912–1923. [Google Scholar] [CrossRef]
- Bhuvaneshwari, S.; Gopalakrishnan, N. Hydrothermally synthesized Copper Oxide (CuO) superstructures for ammonia sensing. J. Colloid Interface Sci. 2016, 480, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonavita, A.; Bonyani, M.; Leonardi, S.G.; Neri, G. Synthesis, Characterization and Gas Sensing Properties of Ag@alpha-Fe2O3 Core-Shell Nanocomposites. Nanomaterials 2015, 5, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Liu, X.; Chen, N.; Deng, D.; Xiao, X.; Wang, Y. A highly sensitive VOC gas sensor using p-type mesoporous Co3O4 nanosheets prepared by a facile chemical coprecipitation method. Sens. Actuators B 2016, 233, 615–623. [Google Scholar] [CrossRef]
- Yang, C.; Deng, W.; Liu, H.; Ge, S.; Yan, M. Turn-on fluorescence sensor for glutathione in aqueous solutions using carbon dots-MnO2 nanocomposites. Sens. Actuators B 2015, 216, 286–292. [Google Scholar] [CrossRef]
- An, X.; Yu, J.C.; Wang, Y.; Hu, Y.; Yu, X.; Zhang, G. WO3 nanorods/graphene nanocomposites for high-efficiency visible-light-driven photocatalysis and NO2 gas sensing. J. Mater. Chem. 2012, 22, 8525–8531. [Google Scholar] [CrossRef]
- Xue, P.; Yang, X.; Lai, X.; Xia, W.; Li, P.; Fang, J. Controlling synthesis and gas-sensing properties of ordered mesoporous In2O3-reduced graphene oxide (rGO) nanocomposite. Sci. Bull. 2015, 60, 1348–1354. [Google Scholar] [CrossRef]
- Lin, L.; Liu, T.; Yu, W.; Guo, Z.; Zeng, W. Synthesis of multifarious hierarchical flower-like NiO and their gas-sensing properties. Mater. Res. Bull. 2013, 48, 2730–2736. [Google Scholar] [CrossRef]
- Choi, K.S.; Park, S.; Chang, S.-P. Enhanced ethanol sensing properties based on SnO2 nanowires coated with Fe2O3 nanoparticles. Sens. Actuators B 2017, 238, 871–879. [Google Scholar] [CrossRef]
- Li, Y.; Chen, N.; Deng, D.; Xing, X.; Xiao, X.; Wang, Y. Formaldehyde detection: SnO2 microspheres for formaldehyde gas sensor with high sensitivity, fast response/recovery and good selectivity. Sens. Actuators B 2017, 238, 264–273. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, A.; Chang, H.; Xia, B. Room-temperature high-performance acetone gas sensor based on hydrothermal synthesized SnO2-reduced graphene oxide hybrid composite. RSC Adv. 2015, 5, 3016–3022. [Google Scholar] [CrossRef]
- Srivastava, V.; Jain, K. At room temperature graphene/SnO2 is better than MWCNT/SnO2 as NO2 gas sensor. Mater. Lett. 2016, 169, 28–32. [Google Scholar] [CrossRef]
- Guo, D.; Cai, P.; Sun, J.; He, W.; Wu, X.; Zhang, T.; Wang, X.; Zhang, X. Reduced-graphene-oxide/metal-oxide p-n heterojunction aerogels as efficient 3D sensing frameworks for phenol detection. Carbon 2016, 99, 571–578. [Google Scholar] [CrossRef]
- Latif, U.; Dickert, F.L. Graphene Hybrid Materials in Gas Sensing Applications. Sensors 2015, 15, 30504–30524. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Zhu, G.; Sun, H.; Sun, J.; Wu, X. Nitrogen-doped hollow carbon spheres wrapped with graphene nanostructure for highly sensitive electrochemical sensing of parachlorophenol. Biosens. Bioelectron. 2016, 86, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Wei, Z.; Wang, B.; Luo, Z.; Xu, S.; Zhang, W.; Yu, H.; Li, M.; Huang, Z.; Zang, J.; et al. Sensitive Room-Temperature H2S Gas Sensors Employing SnO2 Quantum Wire/Reduced Graphene Oxide Nanocomposites. Chem. Mater. 2016, 28, 1205–1212. [Google Scholar] [CrossRef]
- Mao, S.; Cui, S.; Lu, G.; Yu, K.; Wen, Z.; Chen, J. Tuning gas-sensing properties of reduced graphene oxide using tin oxide nanocrystals. J. Mater. Chem. 2012, 22, 11009–11013. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, J.; Fei, T.; Liu, S.; Zhang, T. SnO2 nanoparticles-reduced graphene oxide nanocomposites for NO2 sensing at low operating temperature. Sens. Actuators B 2014, 190, 472–478. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Chang, H.; Liu, A.; Xia, B. Characterization of a hybrid composite of SnO2 nanocrystal-decorated reduced graphene oxide for ppm-level ethanol gas sensing application. RSC Adv. 2015, 5, 18666–18672. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A.K. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Shao, L.; Jiang, D.; Xiao, P.; Zhu, L.; Meng, S.; Chen, M. Enhancement of g-C3N4 nanosheets photocatalysis by synergistic interaction of ZnS microsphere and RGO inducing multistep charge transfer. Appl. Catal. B 2016, 198, 200–210. [Google Scholar] [CrossRef]
- Hu, Y.; Li, L.; Zhang, L.; Lv, Y. Dielectric barrier discharge plasma-assisted fabrication of g-C3N4-Mn3O4 composite for high-performance cataluminescence H2S gas sensor. Sens. Actuators B 2017, 239, 1177–1184. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, S.; Xu, G.; Peng, Y.; Gong, L.; Li, X.; Li, Y.; Lin, Y.; Chen, G. Highly photoactive heterojunction based on g-C3N4 nanosheets decorated with dendritic zinc(II) phthalocyanine through axial coordination and its ultrasensitive enzyme-free sensing of choline. RSC Adv. 2014, 4, 58226–58230. [Google Scholar] [CrossRef]
- Chen, P.W.; Li, K.; Yu, Y.X.; Zhang, W.D. Cobalt-doped graphitic carbon nitride photocatalysts with high activity for hydrogen evolution. Appl. Surf. Sci. 2017, 392, 608–615. [Google Scholar] [CrossRef]
- Wei, J.; Huang, C.; Wu, H.; Kan, E. High-capacity hydrogen storage in Li-adsorbed g-C3N4. Mater. Chem. Phys. 2016, 180, 440–444. [Google Scholar] [CrossRef]
- Carvalho, K.T.G.; Nogueira, A.E.; Lopes, O.F.; Byzynski, G.; Ribeiro, C. Synthesis of g-C3N4/Nb2O5 heterostructures and their application in the removal of organic pollutants under visible and ultraviolet irradiation. Ceram. Int. 2017, 43, 3521–3530. [Google Scholar] [CrossRef]
- Zeng, B.; Zhang, L.; Wan, X.; Song, H.; Lv, Y. Fabrication of α-Fe2O3/g-C3N4, composites for cataluminescence sensing of H2S. Sens. Actuators B 2015, 211, 370–376. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zheng, C.; Guo, S.S.; Li, J.; Yang, H.H.; Chen, G. Turn-on fluorescence sensor for intracellular imaging of glutathione using g-C3N4 nanosheet-MnO2 sandwich nanocomposite. Anal. Chem. 2014, 86, 3426–3434. [Google Scholar] [CrossRef] [PubMed]
- She, X.; Xu, H.; Wang, H.; Xia, J.; Song, Y.; Yan, J.; Xu, Y.; Zhang, Q.; Du, D.; Li, H. Controllable synthesis of CeO2/g-C3N4 composites and their applications in the environment. Dalton Trans. 2015, 44, 7021–7031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, D.; Guo, W.; Chen, S. The α-Fe2O3/g-C3N4 heterostructural nanaocomposites with enhanced ethanol gas sensing performance. J. Alloy. Compd. 2016, 685, 84–90. [Google Scholar] [CrossRef]
- Zito, C.A.; Perfecto, T.M.; Volanti, D.P. Impact of reduced graphene oxide on the ethanol sensing performance of hollow SnO2 nanoparticles under humid atmosphere. Sens. Actuators B 2017, 244, 466–474. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, J.; Gong, H.; Ju, D.; Cao, B. Au nanoparticle-functionalized 3D SnO2, microstructures for high performance gas sensor. Sens. Actuators B 2016, 226, 266–272. [Google Scholar] [CrossRef]
- Zang, Y.; Li, L.; Li, X.; Lin, R.; Li, G. Synergistic collaboration of g-C3N4/SnO2 composites for enhanced visible-light photocatalytic activity. Chem. Eng. J. 2014, 246, 277–286. [Google Scholar] [CrossRef]











© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, J.; Qin, C.; Wang, Y.; Zhang, B.; Gong, Y.; Zhang, H.; Sun, G.; Bala, H.; Zhang, Z. Calcination Method Synthesis of SnO2/g-C3N4 Composites for a High-Performance Ethanol Gas Sensing Application. Nanomaterials 2017, 7, 98. https://doi.org/10.3390/nano7050098
Cao J, Qin C, Wang Y, Zhang B, Gong Y, Zhang H, Sun G, Bala H, Zhang Z. Calcination Method Synthesis of SnO2/g-C3N4 Composites for a High-Performance Ethanol Gas Sensing Application. Nanomaterials. 2017; 7(5):98. https://doi.org/10.3390/nano7050098
Chicago/Turabian StyleCao, Jianliang, Cong Qin, Yan Wang, Bo Zhang, Yuxiao Gong, Huoli Zhang, Guang Sun, Hari Bala, and Zhanying Zhang. 2017. "Calcination Method Synthesis of SnO2/g-C3N4 Composites for a High-Performance Ethanol Gas Sensing Application" Nanomaterials 7, no. 5: 98. https://doi.org/10.3390/nano7050098
APA StyleCao, J., Qin, C., Wang, Y., Zhang, B., Gong, Y., Zhang, H., Sun, G., Bala, H., & Zhang, Z. (2017). Calcination Method Synthesis of SnO2/g-C3N4 Composites for a High-Performance Ethanol Gas Sensing Application. Nanomaterials, 7(5), 98. https://doi.org/10.3390/nano7050098