Chirality on Amorphous High-Tg Polymeric Nanofilms: Optical Activity Amplification by Thermal Annealing
Abstract
:1. Introduction
2. Results
2.1. UV-Vis Absorption and Chiroptical Properties in the Solid State
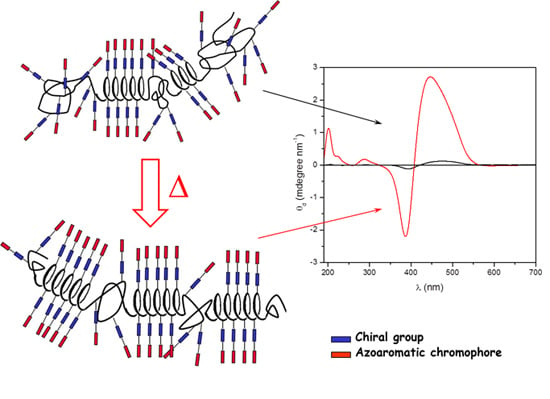
2.2. Annealing
3. Materials and Methods
3.1. Physico-Chemical Measurements
3.2. Materials
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, M.; Asanuma, H.; Komiyama, M. Azobenzene-Tethered T7 Promoter for Efficient Photoregulation of Transcription. J. Am. Chem. Soc. 2006, 128, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Jousselme, B.; Blanchard, P.; Gallego-Planas, N.; Delaunay, J.; Allain, M.; Richomme, P.; Levillain, E.; Roncali, J. Photomechanical Actuation and Manipulation of the Electronic Properties of Linear π-Conjugated Systems. J. Am. Chem. Soc. 2003, 125, 2888–2889. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.-X.; Stellacci, F.; McGrath, D.V. Photoswitchable Flexible and Shape-Persistent Dendrimers: Comparison of the Interplay between a Photochromic Azobenzene Core and Dendrimer Structure. J. Am. Chem. Soc. 2004, 126, 2181–2185. [Google Scholar] [CrossRef] [PubMed]
- Holme, N.C.R.; Nikolova, L.; Norris, T.B.; Hvilsted, S.; Pedersen, M.; Berg, R.H.; Rasmussen, P.H.; Ramanujam, P.S. Physical processes in azobenzene polymers on irradiation with polarized light. Macromol. Symp. 1999, 137, 83–103. [Google Scholar] [CrossRef]
- Green, M.M.; Peterson, N.C.; Sato, T.; Teramoto, A.; Cook, R.; Lifson, S. A helical polymer with a cooperative response to chiral information. Science 1995, 268, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, I.; Ray, S.; Benelli, T.; Raymo, F.M. Dithiolane ligands for semiconductor quantum dots. J. Mater. Chem. 2008, 18, 3940–3947. [Google Scholar] [CrossRef]
- Angiolini, L.; Benelli, T.; Giorgini, L.; Raymo, F.M. Chiroptical switching based on photoinduced proton transfer between homopolymers bearing side-chain spiropyran and azopyridine moieties. Macromol. Chem. Phys. 2008, 209, 2049–2060. [Google Scholar] [CrossRef]
- Holme, N.C.R.; Ramanujam, P.S.; Hvilsted, S. 10000 optical write, read, and erase cycles in an azobenzenesidechain liquid-crystalline polyester. Opt. Lett. 1996, 21, 902–904. [Google Scholar] [CrossRef] [PubMed]
- Benelli, T.; Mazzocchetti, L.; Mazzotti, G.; Paris, F.; Salatelli, E.; Giorgini, L. Supramolecular ordered photochromic cholesteric polymers as smart labels for thermal monitoring applications. Dyes Pigments 2016, 126, 8–19. [Google Scholar] [CrossRef]
- Andruzzi, L.; Altomare, A.; Ciardelli, F.; Solaro, R.; Hvilsted, S.; Ramanujam, P.S. Holographic Gratings in Azobenzene Side-Chain Polymethacrylates. Macromolecules 1999, 32, 448–454. [Google Scholar] [CrossRef]
- Eich, M.; Wendorff, J.H.; Reck, B.; Ringsdorf, H. Reversible digital and holographic optical storage in polymeric liquid crystals. Macromol. Rapid Commun. 1987, 8, 59–63. [Google Scholar] [CrossRef]
- Delaire, J.A.; Nakatani, K. Linear and Nonlinear Optical Properties of Photochromic Molecules and Materials. Chem. Rev. 2000, 100, 1817–1846. [Google Scholar] [CrossRef] [PubMed]
- Mosciatti, T.; Bonacchi, S.; Gobbi, M.; Ferlauto, L.; Liscio, F.; Giorgini, L.; Orgiu, E.; Samorì, P. Optical Input/Electrical Output Memory Elements based on a Liquid Crystalline Azobenzene Polymer. ACS Appl. Mater. Interfaces 2016, 8, 6563–6569. [Google Scholar] [CrossRef] [PubMed]
- Tomatsu, I.; Hashidzume, V.; Harada, A. Contrast Viscosity Changes upon Photoirradiation for Mixtures of Poly(acrylic acid)-based α-Cyclodextrin and Azobenzene Polymers. J. Am. Chem. Soc. 2006, 128, 2226–2227. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, O.; Mitra, N.; Surolia, A.; Jayaraman, N. Photoswitchable Multivalent Sugar Ligands: Synthesis, Isomerization, and Lectin Binding Studies of Azobenzene-Glycopyranoside Derivatives. J. Am. Chem. Soc. 2002, 124, 2124–2125. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Termine, R.; Godbert, N.; Angiolini, L.; Giorgini, L.; Golemme, A. Charge photogeneration and transport in side-chain carbazole polymers and co-polymers. Org. Electron. 2011, 12, 1184–1191. [Google Scholar] [CrossRef]
- Angiolini, L.; Benelli, T.; Giorgini, L.; Golemme, A.; Mazzocchetti, L.; Termine, R. Effect of a chiral substituent on the photochromic and photoconductive properties of a methacrylic polymer bearing side chain azocarbazole moieties. Dyes Pigm. 2014, 102, 53–62. [Google Scholar] [CrossRef]
- Pieroni, O.; Fissi, A.; Popova, G. Photochromic polypeptides. Prog. Polym. Sci. 1998, 23, 81–123. [Google Scholar] [CrossRef]
- Natansohn, A.; Rochon, P.; Ho, M.R.; Barrett, C. Azo Polymers for Reversible Optical Storage. 6. Poly[4-[2-(methacryloyloxy)ethyl]azobenzene]. Macromolecules 1995, 28, 4179–4183. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Q.; Kanazawa, A.; Shiono, T.; Ikeda, T.; Nagase, Y. Photoinduced Alignment of Polymer Liquid Crystals Containing Azobenzene Moieties in the Side Chain. 5. Effect of the Azo Contents on Alignment Behavior and Enhanced Response. Macromolecules 1999, 32, 3951–3956. [Google Scholar] [CrossRef]
- Maertens, C.; Dubois, P.; Jerome, R.; Blanche, P.A.; Lemaire, P.C.J. Synthesis and polarized light-induced birefringence of new polymethacrylates containing carbazolyl and azobenzene pendant groups. Polym. Sci. Part B 2000, 38, 205–213. [Google Scholar] [CrossRef]
- Mendonca, C.R.; Dhanabalan, A.; Balogh, D.T.; Misoguti, L.; Dos Santos, D.S., Jr.; Pereira-da-Silva, M.A.; Giacometti, J.A.; Zilio, S.C.; Oliveira, O.N., Jr. Optically Induced Birefringence and Surface Relief Gratings in Composite Langmuir-Blodgett (LB) Films of Poly[4′-[[2-(methacryloyloxy)ethyl]ethylamino]-2-chloro-4-nitroazobenzene] (HPDR13) and Cadmium Stearate. Macromolecules 1999, 32, 1493–1499. [Google Scholar] [CrossRef]
- Han, M.; Morine, S.; Ichimura, K. Factors Affecting In-Plane and Out-of-Plane Photoorientation of Azobenzene Side Chains Attached to Liquid Crystalline Polymers Induced by Irradiation with Linearly Polarized Light. Macromolecules 2000, 33, 6360–6371. [Google Scholar] [CrossRef]
- Angiolini, L.; Benelli, T.; Giorgini, L.; Paris, F.; Salatelli, E.; Fontana, M.P.; Camorani, P. Synthesis by ATRP and effects of molecular weight on photomechanical properties of liquid crystalline polymers containing side-chain azobenzenechromophore. Eur. Polym. J. 2008, 44, 3231–3238. [Google Scholar] [CrossRef]
- Amouri, H.; Gruselle, M. Chirality in Transition Metal Chemistry: Molecules, Supramolecular Assemblies and Materials; John Wiley & Sons Ltd.: Chichester, UK, 2008. [Google Scholar]
- Angiolini, L.; Benelli, T.; Giorgini, L.; Mauriello, F.; Salatelli, E. Chiroptical and optical thermoplastic acid sensors based on chiral methacrylic polymers containing azoaromatic moieties. Sens. Actuators B 2007, 126, 56–61. [Google Scholar] [CrossRef]
- Angiolini, L.; Benelli, T.; Giorgini, L.; Mauriello, F.; Salatelli, E.; Bozio, R.; Daurù, A.; Pedron, D. Synthesis, chiroptical properties and photoinduced birefringence of optically active methacrylic copolymers bearing side-chain bisazoaromatic moieties. Eur. Polym. J. 2007, 43, 3550–3561. [Google Scholar] [CrossRef]
- Kajitani, T.; Okoshi, K.; Sakurai, S.; Kumaki, J.; Yashima, E. Helix-Sense Controlled Polymerization of a Single Phenyl Isocyanide Enantiomer Leading to Diastereomeric Helical Polyisocyanides with Opposite Helix-Sense and Cholesteric Liquid Crystals with Opposite Twist-Sense. J. Am. Chem. Soc. 2006, 128, 708–709. [Google Scholar] [CrossRef] [PubMed]
- Yashima, E.; Maeda, K.; Nishimura, T. Detection and amplification of chirality by helical polymers. Chem. Eur. J. 2004, 10, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.J.; Masuda, M.; Sijbesma, R.P.; Meijer, E.W. Chiral amplification in the transcription of supramolecularhelicity into a polymer backbone. Angew. Chem. Int. Ed. 2005, 44, 2275–2279. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Liu, R.; Kang, H.; Gao, X.; Shen, D.; Huang, Y. Chirality amplification of syndiotactic polystyrene induced circular dichroism chiral film in δ form upon annealing. Polymer 2011, 52, 3671–3676. [Google Scholar] [CrossRef]
- Cheon, K.S.; Selinger, J.V.; Green, M.M. Designing a Helical Polymer that Reverses its Hendedness at a Selected, Continuously Variable, Temperature. Angew. Chem. Int. Ed. 2000, 39, 1482–1485. [Google Scholar] [CrossRef]
- Rizzo, P.; Guerra, G. Intense Chiral Optical Phenomena in Racemic Polymers by Cocrystallizationwith Chiral Guest Molecules: A Brief Overview. Chirality 2016, 28, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Guerra, G.; Rizzo, P. Racemic synthetic polymers and chirality. Rend. Fis. Acc. Lincei 2013, 24, 217–226. [Google Scholar] [CrossRef]
- Mayer, S.; Maxein, G.; Zentel, R. Photosensitive chiral polyisocyanates. Macromol. Symp. 1999, 137, 67–73. [Google Scholar] [CrossRef]
- Li, J.; Schuster, G.B.; Cheon, K.-S.; Green, M.M.; Selinger, J.V. Switching a Helical Polymer between Mirror Images Using Circularly Polarized Light. J. Am. Chem. Soc. 2000, 122, 2603–2612. [Google Scholar] [CrossRef]
- Ichimura, K. Photoalignment of Liquid-Crystal Systems. Chem. Rev. 2000, 100, 1847–1874. [Google Scholar] [CrossRef] [PubMed]
- Barberá, J.; Giorgini, L.; Paris, F.; Salatelli, E.; Tejedor, R.M.; Angiolini, L. Supramolecular chirality and reversible chiroptical switching in new chiral liquid-crystal azopolymers. Chem. Eur. J. 2008, 14, 11209–11221. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, L.; Todorov, T.; Ivanov, M.; Andruzzi, F.; Hvilsted, S.; Ramanujam, P.S. Photoinduced circular anisotropy in side-chain azobenzene polyesters. Opt. Mater. 1997, 8, 255–258. [Google Scholar] [CrossRef]
- Ivanov, M.; Naydenova, I.; Todorov, T.; Nikolova, L.; Petrova, T.; Tomova, N.; Dragostinova, V. Light-induced optical activity in optically ordered amorphous side-chain azobenzene containing polymer. J. Mod. Opt. 2000, 47, 861–867. [Google Scholar] [CrossRef]
- Iftime, G.; Labarthet, F.L.; Natansohn, A.; Rochon, P. Control of Chirality of an Azobenzene Liquid Crystalline Polymer with Circularly Polarized Light. J. Am. Chem. Soc. 2000, 122, 12646–12650. [Google Scholar] [CrossRef]
- Pagès, S.; Labarthet, F.L.; Buffeteau, T.; Sourisseau, C. Photoinduced linear and/or circular birefringences from light propagation through amorphous or smecticazopolymer films. Appl. Phys. B 2002, 75, 541–548. [Google Scholar]
- Angiolini, L.; Bozio, R.; Giorgini, L.; Pedron, D.; Turco, G.; Daurù, A. Photomodulation of the Chiroptical Properties of New Chiral Methacrylic Polymers with Side Chain Azobenzene Moieties. Chem. Eur. J. 2002, 8, 4241–4247. [Google Scholar] [CrossRef]
- Angiolini, L.; Benelli, T.; Bozio, R.; Daurù, A.; Giorgini, L.; Pedron, D. Photoinduced chiroptical bistability in new chiral methacrylic azobenzene-containing polymers. Synth. Met. 2003, 139, 743–746. [Google Scholar] [CrossRef]
- Angiolini, L.; Giorgini, L.; Bozio, R.; Pedron, D. Reversible chirality inversion of photochromic methacrylic polymers upon irradiation with one-handed circularly polarized light. Synth. Met. 2003, 138, 375–379. [Google Scholar] [CrossRef]
- Ohira, A.; Okoshi, K.; Fujiki, M.; Kunitake, M.; Naito, M.; Hogihara, T. Versatile Helical Polymer Films: Chiroptical Inversion Switching and Memory with Re-Writable (RW) and Write-Once Read-Many (WORM) Modes. Adv. Mater. 2004, 16, 1645–1650. [Google Scholar] [CrossRef]
- Angiolini, L.; Caretti, D.; Giorgini, L.; Salatelli, E. Methacrylic polymers bearing side-chain permanent dipole azobenzene chromophores spaced from the main chain by chiral moieties: Synthesis and characterization. Polymer 2001, 42, 4005–4016. [Google Scholar] [CrossRef]
- Angiolini, L.; Benelli, T.; Giorgini, L.; Salatelli, E.; Bozio, R.; Daurù, A.; Pedron, D. Synthesis, chiroptical properties and photoinduced linear birefringence of the homopolymer of (R)-3-methacryloyloxy-1-(4’-cyano-4-azobenzene) pyrrolidine and of the copolymers with the enantiomeric monomer. Eur. Polym. J. 2005, 41, 2045–2054. [Google Scholar] [CrossRef]
- Angiolini, L.; Benelli, T.; Giorgini, L.; Salatelli, E. Optically active photochromic methacrylic polymers with controlled average molecular weight and defined end-groups by atom transfer radical polymerization. Polymer 2005, 46, 2424–2432. [Google Scholar] [CrossRef]
- Angiolini, L.; Benelli, T.; Giorgini, L.; Salatelli, E. Synthesis of optically active methacrylicoligomeric models and polymers bearing the side-chain azo-aromatic moiety and dependence of their chiroptical properties on the polymerization degree. Polymer 2006, 47, 1875–1885. [Google Scholar] [CrossRef]
- Painelli, A.; Terenziani, F.; Angiolini, L.; Benelli, T.; Giorgini, L. Chiral Interactions in Azobenzene Dimers: A Combined Experimental and Theoretical Study. Chem. Eur. J. 2005, 11, 6053–6063. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, M.; Kunitake, T. Fluorescence and photoisomerization of azobenzene-containing bilayer membranes. J. Am. Chem. Soc. 1987, 109, 5175–5183. [Google Scholar] [CrossRef]
- Menzel, H.; Weichart, B.; Schmidt, A.; Paul, S.; Knoll, W.; Stumpe, J.; Fischer, T. Small-Angle X-ray Scattering and Ultraviolet-Visible Spectroscopy Studies on the Structure and Structural Changes in Langmuir-Blodgett Films of Polyglutamates with Azobenzene Moieties Tethered by Alkyl Spacers of Different Length. Langmuir 1994, 10, 1926–1933. [Google Scholar] [CrossRef]
- Dhanabalan, A.; Balogh, D.T., Jr.; Riul, A.; Giacometti, J.A.; Oliveira, O.N., Jr. Langmuir and Langmuir–Blodgett films of a homopolymer of Disperse Red-13. Thin Solid Films 1998, 323, 257–264. [Google Scholar] [CrossRef]
- Verbiest, T.; Kauranen, M.; Persoon, A. Second-order nonlinear optical properties of chiral thin films. J. Mater. Chem. 1999, 9, 2005–2012. [Google Scholar] [CrossRef]
- Rodger, A.; Nord’en, B. Circular Dichroism, and Linear Dichroism; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Saxena, A.; Guo, G.; Fujiki, M.; Yang, Y.; Ohira, A.; Okoshi, K.; Naito, M. Helical Polymer Command Surface: Thermodriven Chiroptical Transfer and Amplification in Binary Polysilane Film System. Macromolecules 2004, 37, 3081–3083. [Google Scholar] [CrossRef]
- Zou, G.; Wang, Y.; Zhang, Q.; Kohn, H.; Manaka, T.; Iwamoto, M. Molecular structure modulated properties of azobenzene-substituted polydiacetylene LB films: Chirality formation and thermal stability. Polymer 2010, 51, 2229–2235. [Google Scholar] [CrossRef]
- Mason, S.F. Molecular Optical Activity and the Chiral Discrimination; Cambridge University Press: Cambridge, UK, 1982; Chapter 3. [Google Scholar]
- Berova, N.; Nakanishi, K.; Woody, R.W. Circular Dichroism, Principles and Applications; Wiley–VCH: New York, NY, USA, 2000. [Google Scholar]








| Sample | λmaxa (εmax10−3) b | λmaxa (εmax10−3) b |
|---|---|---|
| Poly[(R)-MAP-C] DMA c | 450 (33.3) | 276 (13.3) |
| Poly[(R)-MAP-C] film | 424 | 276 |
| copol (R)-75 DMA c | 449 (33.6) | 273 (14.4) |
| copol (R)-75 film | 425 | 276 |
| copol (rac) DMA c | 448 (35.0) | 277 (13.7) |
| copol (rac) film | 425 | 276 |
| copol (S)-75 DMA c | 448 (34.8) | 276 (14.0) |
| copol (S)-75 film | 424 | 276 |
| Poly[(S)-MAP-C] DMA d | 447 (32.4) | 277 (12.4) |
| Poly[(S)-MAP-C] film | 426 | 275 |
| (S)-PAP-C DMA d | 458 (35.4) | 277 (12.5) |
| Sample | 1st Absorption Band | 2nd Absorption Band | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| λ1 a | θd1 b | λ0 c | λ2 a | θd2 b | λ3 a | θd3 b | λ4 c | λ5 a | θd5 b | |
| Poly[(R)-MAP-C] | 463 | ‒0.17 | 417 | 392 | +0.16 | 292 | ‒0.002 | 282 | 262 | +0.012 |
| Poly[(R)-MAP-C] ann | 438 | ‒4.65 | 403 | 387 | +2.46 | 282 | ‒0.53 | 261 | 253 | +0.053 |
| copol (R)-75 | 460 | ‒0.10 | 418 | 391 | +0.11 | 293 | ‒0.001 | 288 | 264 | +0.010 |
| copol (rac) | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| copol (rac) ann | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ | ‒ |
| copol (S)-75 | 461 | +0.14 | 415 | 390 | ‒0.13 | 295 | +0.008 | 277 | 261 | ‒0.007 |
| Poly[(S)-MAP-C] | 475 | +0.16 | 421 | 393 | ‒0.13 | 291 | +0.003 | 281 | 264 | ‒0.011 |
| Poly[(S)-MAP-C] ann | 446 | +2.71 | 408 | 387 | ‒2.20 | 288 | +0.18 | 266 | 259 | ‒0.017 |
| Sample | Feed in mol % | Mn a | Mw/Mn a | Tg (°C) b | |
|---|---|---|---|---|---|
| (R)-MAP-C | (S)-MAP-C | ||||
| Poly[(R)-MAP-C] | 100c | 0 | 32,900 | 1.5 | 185 |
| copol (R)-75 | 75c | 25 | 33,200 | 1.5 | 190 |
| copol (rac) | 50c | 50 | 33,600 | 1.4 | 192 |
| copol (S)-75 | 25c | 75 | 31,800 | 1.5 | 190 |
| Poly[(S)-MAP-C] | 0 | 100 | 43,900 | 1.4 | 192 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benelli, T.; Lanzi, M.; Mazzocchetti, L.; Giorgini, L. Chirality on Amorphous High-Tg Polymeric Nanofilms: Optical Activity Amplification by Thermal Annealing. Nanomaterials 2017, 7, 208. https://doi.org/10.3390/nano7080208
Benelli T, Lanzi M, Mazzocchetti L, Giorgini L. Chirality on Amorphous High-Tg Polymeric Nanofilms: Optical Activity Amplification by Thermal Annealing. Nanomaterials. 2017; 7(8):208. https://doi.org/10.3390/nano7080208
Chicago/Turabian StyleBenelli, Tiziana, Massimiliano Lanzi, Laura Mazzocchetti, and Loris Giorgini. 2017. "Chirality on Amorphous High-Tg Polymeric Nanofilms: Optical Activity Amplification by Thermal Annealing" Nanomaterials 7, no. 8: 208. https://doi.org/10.3390/nano7080208
APA StyleBenelli, T., Lanzi, M., Mazzocchetti, L., & Giorgini, L. (2017). Chirality on Amorphous High-Tg Polymeric Nanofilms: Optical Activity Amplification by Thermal Annealing. Nanomaterials, 7(8), 208. https://doi.org/10.3390/nano7080208