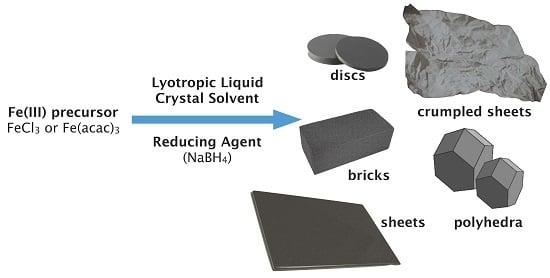
Synthesis of Distinct Iron Oxide Nanomaterial Shapes Using Lyotropic Liquid Crystal Solvents
Abstract
:1. Introduction
1.1. Shape Effects on Particle Translocation and Endocytosis
1.2. The Influence of Iron Oxide Nanoparticle Shape
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kurzhals, S.; Gal, N.; Zirbs, R.; Reimhult, E. Controlled aggregation and cell uptake of thermoresponsive polyoxazoline-grafted superparamagnetic iron oxide nanoparticles. Nanoscale 2017, 9, 2793–2805. [Google Scholar] [CrossRef] [PubMed]
- Lassenberger, A.; Scheberl, A.; Stadlbauer, A.; Stiglbauer, A.; Helbich, T.; Reimhult, E. Individually stabilized, superparamagnetic nanoparticles with controlled shell and size leading to exceptional stealth properties and high relaxivities. ACS Appl. Mater. Interfaces 2017, 9, 3343–3353. [Google Scholar] [CrossRef] [PubMed]
- Gal, N.; Lassenberger, A.; Herrero-Nogareda, L.; Scheberl, A.; Charwat, V.; Kasper, C.; Reimhult, E. Interaction of size-tailored pegylated iron oxide nanoparticles with lipid membranes and cells. ACS Biomater. Sci. Eng. 2017, 3, 249–259. [Google Scholar] [CrossRef]
- Ghosh, D.; Lee, Y.; Thomas, S.; Kohli, A.G.; Yun, D.S.; Belcher, A.M.; Kelly, K.A. M13-templated magnetic nanoparticles for targeted in vivo imaging of prostate cancer. Nat. Nanotechnol. 2012, 7, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, F.; Davies, G.L.; Monopoli, M.P.; Moloney, M.; Gun’ko, Y.K.; Salvati, A.; Dawson, K.A. Magnetic nanoparticles to recover cellular organelles and study the time resolved nanoparticle-cell interactome throughout uptake. Small 2014, 10, 3307–3315. [Google Scholar] [CrossRef] [PubMed]
- Basuki, J.S.; Esser, L.; Duong, H.T.T.; Zhang, Q.; Wilson, P.; Whittaker, M.R.; Haddleton, D.M.; Boyer, C.; Davis, T.P. Magnetic nanoparticles with diblock glycopolymer shells give lectin concentration-dependent mri signals and selective cell uptake. Chem. Sci. 2014, 5, 715–726. [Google Scholar] [CrossRef]
- Grafe, C.; Slabu, I.; Wiekhorst, F.; Bergemann, C.; von Eggeling, F.; Hochhaus, A.; Trahms, L.; Clement, J.H. Magnetic particle spectroscopy allows precise quantification of nanoparticles after passage through human brain microvascular endothelial cells. Phys. Med. Biol. 2016, 61, 3986–4000. [Google Scholar] [CrossRef] [PubMed]
- Davila-Ibanez, A.B.; Salgueirino, V.; Martinez-Zorzano, V.; Marino-Fernandez, R.; Garcia-Lorenzo, A.; Maceira-Campos, M.; Munoz-Ubeda, M.; Junquera, E.; Aicart, E.; Rivas, J.; et al. Magnetic silica nanoparticle cellular uptake and cytotoxicity regulated by electrostatic polyelectrolytes-DNA loading at their surface. ACS Nano 2012, 6, 747–759. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, D.; Tenzer, S.; Bannwarth, M.B.; Messerschmidt, C.; Glaser, S.F.; Schild, H.; Landfester, K.; Mailander, V. Mass spectrometry and imaging analysis of nanoparticle-containing vesicles provide a mechanistic insight into cellular trafficking. ACS Nano 2014, 8, 10077–10088. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.; Gazeau, F.; Bacri, J.C.; Wilhelm, C.; Devaud, M. Modeling magnetic nanoparticle dipole-dipole interactions inside living cells. Phys. Rev. B 2011, 84, 075480. [Google Scholar] [CrossRef]
- Lunov, O.; Zablotskii, V.; Syrovets, T.; Rocker, C.; Tron, K.; Nienhaus, G.U.; Simmet, T. Modeling receptor-mediated endocytosis of polymer-functionalized iron oxide nanoparticles by human macrophages. Biomaterials 2011, 32, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.R.; Chari, D.M. A multicellular, neuro-mimetic model to study nanoparticle uptake in cells of the central nervous system. Integr. Biol. 2014, 6, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Bothun, G.D.; Lelis, A.; Chen, Y.J.; Scully, K.; Anderson, L.E.; Stoner, M.A. Multicomponent folate-targeted magnetoliposomes: Design, characterization, and cellular uptake. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Liong, M.; Lu, J.; Kovochich, M.; Xia, T.; Ruehm, S.G.; Nel, A.E.; Tamanoi, F.; Zink, J.I. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2008, 2, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Lartigue, L.; Wilhelm, C.; Servais, J.; Factor, C.; Dencausse, A.; Bacri, J.C.; Luciani, N.; Gazeau, F. Nanomagnetic sensing of blood plasma protein interactions with iron oxide nanoparticles: Impact on macrophage uptake. ACS Nano 2012, 6, 2665–2678. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.N.; Deng, Z.Y.; Liu, G.H.; Hu, J.M.; Liu, S.Y. Photoregulated cross-linking of superparamagnetic iron oxide nanoparticle (spion) loaded hybrid nanovectors with synergistic drug release and magnetic resonance (MR) imaging enhancement. Macromolecules 2017, 50, 1113–1125. [Google Scholar] [CrossRef]
- Sherlock, S.P.; Tabakman, S.M.; Xie, L.M.; Dai, H.J. Photothermally enhanced drug delivery by ultrasmall multifunctional feco/graphitic shell nanocrystals. ACS Nano 2011, 5, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.L.; Gao, H.J.; Bao, G. Physical principles of nanoparticle cellular endocytosis. ACS Nano 2015, 9, 8655–8671. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Hurley, K.R.; Bischof, J.C.; Haynes, C.L.; Hogan, C.J. Quantifying intra- and extracellular aggregation of iron oxide nanoparticles and its influence on specific absorption rate. Nanoscale 2016, 8, 16053–16064. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Mal, N.; Williams, P.S.; Mayorga, M.; Penn, M.S.; Chalmers, J.J.; Zborowski, M. Quantitative intracellular magnetic nanoparticle uptake measured by live cell magnetophoresis. FASEB J. 2008, 22, 4239–4247. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Naregalkar, R.R.; Vaidya, V.D.; Gupta, M. Recent advances on surface engineering of magnetic iron oxide nanoparticles and their biomedical applications. Nanomedicine 2007, 2, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.L.; Sherwood, J.A.; Lackey, K.H.; Qin, Y.; Bao, Y.P. The responses of immune cells to iron oxide nanoparticles. J. Appl. Toxicol. 2016, 36, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, J.; Lovas, K.; Rich, M.; Yin, Q.; Lackey, K.; Bolding, M.S.; Bao, Y. Shape-dependent cellular behaviors and relaxivity of iron oxide-based T-1 mri contrast agents. Nanoscale 2016, 8, 17506–17515. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, V.; Kinnear, C.; Moniatte, M.; Rothen-Rutishauser, B.; Clift, M.J.D.; Fink, A. Surface charge of polymer coated spions influences the serum protein adsorption, colloidal stability and subsequent cell interaction in vitro. Nanoscale 2013, 5, 3723–3732. [Google Scholar] [CrossRef] [PubMed]
- Mo, J.B.; He, L.Z.; Ma, B.; Chen, T.F. Tailoring particle size of mesoporous silica nanosystem to antagonize glioblastoma and overcome blood-brain barrier. ACS Appl. Mater. Interfaces 2016, 8, 6811–6825. [Google Scholar] [CrossRef] [PubMed]
- Kievit, F.M.; Stephen, Z.R.; Veiseh, O.; Arami, H.; Wang, T.Z.; Lai, V.P.; Park, J.O.; Ellenbogen, R.G.; Disis, M.L.; Zhang, M.Q. Targeting of primary breast cancers and metastases in a transgenic mouse model using rationally designed multifunctional spions. ACS Nano 2012, 6, 2591–2601. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.J.; Miranda-Nieves, D.; Ankrum, J.A.; Matthiesen, M.E.; Phillips, J.A.; Roes, I.; Wojtkiewicz, G.R.; Juneja, V.; Kultima, J.R.; Zhao, W.A.; et al. Tracking mesenchymal stem cells with iron oxide nanoparticle loaded poly(lactide-co-glycolide) microparticles. Nano Lett. 2012, 12, 4131–4139. [Google Scholar] [CrossRef] [PubMed]
- Massart, R. Preparation of aqueous magnetic liquids in alkaline and acidic media. IEEE Trans. Magn. 1981, 17, 1247–1248. [Google Scholar] [CrossRef]
- Sun, S.H.; Zeng, H. Size-controlled synthesis of magnetite nanoparticies. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Garcia, A.; Sun, S.H. Recent advances in the high-temperature chemical synthesis of magnetic nanoparticles. Adv. Funct. Mater. 2016, 26, 3809–3817. [Google Scholar] [CrossRef]
- Song, M.M.; Song, W.J.; Bi, H.; Wang, J.; Wu, W.L.; Sun, J.; Yu, M. Cytotoxicity and cellular uptake of iron nanowires. Biomaterials 2010, 31, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Gil, S.; Correia, C.R.; Mano, J.F. Magnetically labeled cells with surface-modified Fe3O4 spherical and rod-shaped magnetic nanoparticles for tissue engineering applications. Adv. Healthc. Mater. 2015, 4, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Xia, X.M.; Wu, M.; Cui, C.; Zhang, Y.; Liu, L.; Wu, B.; Wang, C.X.; Zhang, L.J.; Zhou, X.; et al. Folic acid-conjugated iron oxide porous nanorods loaded with doxorubicin for targeted drug delivery. Coll. Surf. B 2014, 120, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.G.; Wei, W.; You, Z.X.; Yang, Q.Z.; Yue, H.; Su, Z.G.; Ma, G.H. Iron oxide nanotubes for magnetically guided delivery and pH-activated release of insoluble anticancer drugs. Adv. Funct. Mater. 2011, 21, 3446–3453. [Google Scholar] [CrossRef]
- Safi, M.; Yan, M.H.; Guedeau-Boudeville, M.A.; Conjeaud, H.; Garnier-Thibaud, V.; Boggetto, N.; Baeza-Squiban, A.; Niedergang, F.; Averbeck, D.; Berret, J.F. Interactions between magnetic nanowires and living cells: Uptake, toxicity, and degradation. ACS Nano 2011, 5, 5354–5364. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.C.; Wang, W.S.; Huang, Y.T.; Sheu, H.S.; Lo, Y.W.; Tsai, T.L.; Shieh, D.B.; Yeh, C.S. Porous iron oxide based nanorods developed as delivery nanocapsules. Chem. Eur. J. 2007, 13, 3878–3885. [Google Scholar] [CrossRef] [PubMed]
- Key, J.; Dhawan, D.; Cooper, C.L.; Knapp, D.W.; Kim, K.; Kwon, I.C.; Choi, K.; Park, K.; Decuzzi, P.; Leary, J.F. Multicomponent, peptide-targeted glycol chitosan nanoparticles containing ferrimagnetic iron oxide nanocubes for bladder cancer multimodal imaging. Int. J. Nanomed. 2016, 11, 4141–4155. [Google Scholar] [CrossRef] [PubMed]
- Wortmann, L.; Ilyas, S.; Niznansky, D.; Valldor, M.; Arroub, K.; Berger, N.; Rahme, K.; Holmes, J.; Mathur, S. Bioconjugated iron oxide nanocubes: Synthesis, functionalization, and vectorization. ACS Appl. Mater. Interfaces 2014, 6, 16631–16642. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.; Chen, Y.J.; Chen, J.X.; Yang, B.Y.; Zhang, Y.; Gao, H.L.; Hua, Z.C.; Gu, N. Rubik-like magnetic nanoassemblies as an efficient drug multifunctional carrier for cancer theranostics. J. Control. Release 2013, 172, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.Y.; Timmerman, M.; van de Putte, M.; Rodriguez, P.G.; Veldhuis, S.; ten Elshof, J.E. Self-assembly of metal oxide nanosheets at liquid-air interfaces in colloidal solutions. J. Phys. Chem. C 2016, 120, 25411–25417. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Ma, T.C.; Zhao, E.Y.; Docter, D.; Yang, W.S.; Stauber, R.H.; Gao, M.Y. Small is smarter: Nano mri contrast agents-advantages and recent achievements. Small 2016, 12, 556–576. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.Z.; Worden, M.; Wroczynskyj, Y.; Manna, P.K.; Thliveris, J.A.; van Lierop, J.; Hegmann, T.; Miller, D.W. Differential internalization of brick shaped iron oxide nanoparticles by endothelial cells. J. Mater. Chem. B 2016, 4, 5913–5920. [Google Scholar] [CrossRef]
- Vacha, R.; Martinez-Veracoechea, F.J.; Frenkel, D. Receptor-mediated endocytosis of nanoparticles of various shapes. Nano Lett. 2011, 11, 5391–5395. [Google Scholar] [CrossRef] [PubMed]
- Nangia, S.; Sureshkumar, R. Effects of nanoparticle charge and shape anisotropy on translocation through cell membranes. Langmuir 2012, 28, 17666–17671. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tekobo, S.; Tu, Y.; Zhou, Q.F.; Jin, X.L.; Dergunov, S.A.; Pinkhassik, E.; Yan, B. Permission to enter cell by shape: Nanodisk vs. nanosphere. ACS Appl. Mater. Interfaces 2012, 4, 4099–4105. [Google Scholar] [CrossRef] [PubMed]
- Worden, M.; Bruckman, M.A.; Kim, M.H.; Steinmetz, N.F.; Kikkawa, J.M.; LaSpina, C.; Hegmann, T. Aqueous synthesis of polyhedral “brick-like” iron oxide nanoparticles for hyperthermia and t-2 mri contrast enhancement. J. Mater. Chem. B 2015, 3, 6877–6884. [Google Scholar] [CrossRef] [PubMed]
- Yathindranath, V.; Ganesh, V.; Worden, M.; Inokuchi, M.; Hegmann, T. Highly crystalline iron/iron oxide nanosheets via lyotropic liquid crystal templating. RSC Adv. 2013, 3, 9210–9213. [Google Scholar] [CrossRef]
- Worden, M.; Bergquist, L.; Hegmann, T. A quick and easy synthesis of fluorescent iron oxide nanoparticles featuring a luminescent carbonaceous coating via in situ pyrolysis of organosilane ligands. RSC Adv. 2015, 5, 100384–100389. [Google Scholar] [CrossRef]
- Yathindranath, V.; Worden, M.; Sun, Z.Z.; Miller, D.W.; Hegmann, T. A general synthesis of metal (Mn, Fe, Co, Ni, Cu, Zn) oxide and silica nanoparticles based on a low temperature reduction/hydrolysis pathway. RSC Adv. 2013, 3, 23722–23729. [Google Scholar] [CrossRef]
- Yathindranath, V.; Sun, Z.Z.; Worden, M.; Donald, L.J.; Thliveris, J.A.; Miller, D.W.; Hegmann, T. One-pot synthesis of iron oxide nanoparticles with functional silane shells: A versatile general precursor for conjugations and biomedical applications. Langmuir 2013, 29, 10850–10858. [Google Scholar] [CrossRef] [PubMed]
- Yathindranath, V.; Rebbouh, L.; Moore, D.F.; Miller, D.W.; van Lierop, J.; Hegmann, T. A versatile method for the reductive, one-pot synthesis of bare, hydrophilic and hydrophobic magnetite nanoparticles. Adv. Funct. Mater. 2011, 21, 1457–1464. [Google Scholar] [CrossRef]
- Beyer, K. Phase structures, water binding, and molecular-dynamics in liquid-crystalline and frozen states of the system Triton X-100-D2O—A deuteron and carbon NMR-study. J. Coll. Interface Sci. 1982, 86, 73–89. [Google Scholar] [CrossRef]
- Fritscher, C.; Husing, N.; Bernstorff, S.; Brandhuber, D.; Koch, T.; Seidler, S.; Lichtenegger, H.C. In situ saxs study on cationic and non-ionic surfactant liquid crystals using synchrotron radiation. J. Synchrotron Radiat. 2005, 12, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.D.; Zhou, W.J.; Li, H.L. Effects of deposition potential and anneal temperature on the hexagonal nanoporous nickel hydroxide films. Chem. Mater. 2007, 19, 3882–3891. [Google Scholar] [CrossRef]
- Raman, N.K.; Anderson, M.T.; Brinker, C.J. Template-based approaches to the preparation of amorphous, nanoporous silicas. Chem. Mater. 1996, 8, 1682–1701. [Google Scholar] [CrossRef]
- Dale, J. Reduction of symmetrical 1,2- and 1,3-diketones with sodium borohydride, and separation of diastereoisomeric 1,2- and 1,3-diols by means of borate complexes. J. Chem. Soc. 1961, 910–922. [Google Scholar] [CrossRef]












| Surfactant | Iron Precursor/Conc., Phase/wt % Surfactant, Temperature 1 | Main Morphology 2 Gel [Froth] | Dimensions 3 w × d × h (nm) |
|---|---|---|---|
| Triton X-45 | FeCl3/1 mmol, Lα/50, 40 °C | NS [NS + NPs] | 100 × 100 × 5 [20] |
| FeCl3/2 mmol, Lα/50, 40 °C | NS + NPs [NS + NPs] | 300 × 300 × 10 [20] | |
| FeCl3/1 mmol, Lα/20, 40 °C | NP [NS] | 300 × 300 × 10 [20] | |
| Fe(acac)3/1 mmol, Lα/50, 40 °C | NP [NP] | 15–20 [15–20] | |
| Triton X-100 | FeCl3/1 mmol, ColhI/48, 25 °C [47] | NS [NS] | 500+ × 500+ × 5, 10 |
| FeCl3/1 mmol, Lα/70, 3 °C | NScr [NScr] | 100+ × 100+ × 5, 10 | |
| Brij-C10 | FeCl3/1 mmol, Lα/72.5, 62 °C | NScr [NP] | 10 [100+ × 100+ × 5, 10] |
| FeCl3/1 mmol, Lα/55, 85 °C | NP + few NScr [NP] | 40 [40] | |
| CTAB | FeCl3/1 mmol, ColhI/25, 25 °C | NS + NP [ND] | 100 × 100 × 10 [20] 4 |
| Fe(acac)3/1 mmol, ColhI/25, 25 °C | ND [NP] | 20 4 [20] | |
| FeCl3/1 mmol, Isosp-mic/3.6, 50 °C | NS + NP [NScr] | 500+ × 500+ × 10, 20 | |
| FeCl3/1 mmol, Isorl-mic/20, 60 °C | ND [NScr] | 20–30 4 [100+ × 100+ × 20] | |
| Fe(acac)3/1 mmol, Isorl-mic/20, 60 °C | NP [NP] | 10–20 [10–20] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salili, S.M.; Worden, M.; Nemati, A.; Miller, D.W.; Hegmann, T. Synthesis of Distinct Iron Oxide Nanomaterial Shapes Using Lyotropic Liquid Crystal Solvents. Nanomaterials 2017, 7, 211. https://doi.org/10.3390/nano7080211
Salili SM, Worden M, Nemati A, Miller DW, Hegmann T. Synthesis of Distinct Iron Oxide Nanomaterial Shapes Using Lyotropic Liquid Crystal Solvents. Nanomaterials. 2017; 7(8):211. https://doi.org/10.3390/nano7080211
Chicago/Turabian StyleSalili, Seyyed Muhammad, Matthew Worden, Ahlam Nemati, Donald W. Miller, and Torsten Hegmann. 2017. "Synthesis of Distinct Iron Oxide Nanomaterial Shapes Using Lyotropic Liquid Crystal Solvents" Nanomaterials 7, no. 8: 211. https://doi.org/10.3390/nano7080211
APA StyleSalili, S. M., Worden, M., Nemati, A., Miller, D. W., & Hegmann, T. (2017). Synthesis of Distinct Iron Oxide Nanomaterial Shapes Using Lyotropic Liquid Crystal Solvents. Nanomaterials, 7(8), 211. https://doi.org/10.3390/nano7080211