The Effects of Silica Nanoparticles on Apoptosis and Autophagy of Glioblastoma Cell Lines
Abstract
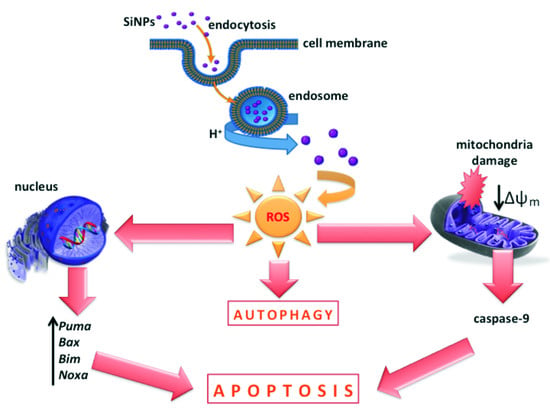
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Cultures and Exposure to Silica Nanoparticles
2.3. Cell Viability
2.4. Characterization of Silica Nanoparticles
2.5. Detection of Apoptosis and Necrosis
2.6. Intracellular ROS Detection
2.7. Mitochondrial Membrane Potential (ΔΨm) Analysis
2.8. Caspase-9 Activity Analysis
2.9. RNA Isolation
2.10. Gene Expression Analysis
2.11. Transmission Electron Microscopy
2.12. Western Analysis
2.13. Protein Assay
2.14. Fluorescent Microscopy Assay
2.15. Statistical Analysis
3. Results
3.1. The Effect of Silica Nanoparticles on Cell Viability
3.2. The Effect of the Dispersion Media on Zeta Potentials
3.3. The Effect of Silica Nanoparticles on Apoptosis and Necrosis
3.4. The Effect of Silica Nanoparticles on Intracellular ROS Generation
3.5. The Effect of Silica Nanoparticles on the Change of Mitochondrial Membrane Potential
3.6. The Effect of Silica Nanoparticles on Caspase-9 Activity
3.7. The Effect of Silica Nanoparticles on Proapoptotic Genes Expression
3.8. The Effect of Silica Nanoparticles on Morphological Changes of LBC3 Cells
3.9. The Effect of Silica Nanoparticles on Expression of Autophagy Markers
3.10. The Effect of Silica Nanoparticles on Acidic Vesicular Organelles Formation
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Compliance with Ethical Standards
Abbreviations
| AVOs | acidic vacuolar organelles |
| DCFH-DA | dichlorodihydrofluorescein diacetate |
| GBM | glioblastoma multiforme |
| HUVECs | human umbilical vein endothelial cells |
| JC-1 | 5,5,6,6-tetrachloro-1,1,3,3-tetraethylbenzimidazolcarbocyanine iodide |
| LC3 | microtubule-associated protein 1A/1B-light chain |
| MMP | mitochondrial membrane potential |
| MPTP | mitochondrial permeability transition pores |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| APAF-1 | apoptotic protease activating factor 1 |
| ROS | reactive oxygen species |
| SiNPs | silica nanoparticles |
References
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Caldarella, A.; Barchielli, A. Glioblastoma in the Canton of Zurich Switzerland revisited: 2005 to 2009. Cancer 2016, 122, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Verma, J.; Lal, S.; Van Noorden, C.J. Nanoparticles for hyperthermic therapy: Synthesis strategies and applications in glioblastoma. Int. J. Nanomed. 2014, 10, 2863–2877. [Google Scholar]
- Yao, K.C.; Komata, T.; Kondo, Y.; Kanzawa, T.; Kondo, S.; Germano, I.M. Molecular response of human glioblastoma multiforme cells to ionizing radiation: Cell cycle arrest modulation of the expression of cyclin-dependent kinase inhibitors and autophagy. J. Neurosurg. 2003, 98, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Mamaeva, V.; Rosenholm, J.M.; Bate-Eya, L.T.; Bergman, L.; Peuhu, E.; Duchanoy, A.; Fortelius, L.E.; Landor, S.; Toivola, D.M.; Lindén, M.; et al. Mesoporous silica nanoparticles as drug delivery systems for targeted inhibition of Notch signaling in cancer. Mol. Ther. 2011, 19, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Petrache Voicu, S.N.; Dinu, D.; Sima, C.; Hermenea, A.; Ardelean, A.; Codrici, E.; Stan, M.S.; Zarnescu, O.; Dinischiotu, A. Silica Nanoparticles Induce Oxidative Stress and Autophagy but Not Apoptosis in the MRC-5 Cell Line. Int. J. Mol. Sci. 2015, 16, 29398–29416. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Sugihara, F.; Matsushita, H.; Yoshioka, Y.; Mizukami, S.; Kikuchi, K. Mesoporous silica nanoparticles for 19F magnetic resonance imaging fluorescence imaging and drug delivery. Chem. Sci. 2015, 6, 1986–1990. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Min, M.S.; Zao, J.X. Recent development of silica nanoparticles as delivery vectors from cancer imaging and therapy. Nanomedicine 2014, 10, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Kempen, P.J.; Greasley, S.; Parker, K.A.; Campbell, J.L.; Chang, H.Y.; Jones, J.R.; Sinclair, R.; Gambhir, S.S.; Jokerst, J.V. Theranostic mesoporous silica nanoparticles biodegrade after pro-survival drug delivery and ultrasound/magnetic resonance imaging of stem cells. Theranostics 2015, 5, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Na, H.K.; Kim, Y.K.; Ryoo, S.R.; Cho, H.S.; Lee, K.E.; Jeon, H.; Ryoo, R.; Min, D.H. Facile synthesis of monodispersed mesoporous silica nanoparticles with ultra large pores and their application in gene delivery. ACS Nano 2011, 5, 3568–3576. [Google Scholar] [CrossRef] [PubMed]
- Arap, W.; Pasqualini, S.; Montalti, M.; Petrizza, L.; Prodi, L.; Rampazzo, E.; Zaccheroni, N.; Marchio, S. Luminescent silica nanoparticles for cancer diagnosis. Curr. Med. Chem. 2013, 20, 2195–2211. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; von Mikecz, A. Formation of nucleoplasmic protein aggregates impairs nuclear function in response to SiO2 nanoparticles. Exp. Cell Res. 2005, 305, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Nabeshi, H.; Yoshikawa, T.; Matsuyama, K.; Nakazato, Y.; Tochigi, S.; Kondoh, S.; Hirai, T.; Akase, T.; Nagano, K.; Abe, Y.; et al. Amorphous nanosilica induce endocytosis-dependent ROS generation and DNA damage in human keratinocytes. Part. Fibre Toxicol. 2011, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.Y.; Joachim, E.; Choi, H.; Kim, K. Toxicity of silica nanoparticles depends on size dose and cell type. Nanomedicine 2015, 11, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Singer, L.; Scharf, A.; von Mikecz, A. Nuclear polyglutamine-containing protein aggregates as active proteolytic centers. J. Cell Biol. 2008, 180, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Xia, Y.; Niu, P.; Jiang, L.; Duan, J.; Yu, Y.; Zhou, X.; Li, Y.; Sun, Z. Silica nanoparticles induce oxidative stress, inflammation, and endothelial dysfunction in vitro viaactivation of the MAPK/Nrf2 pathway and nuclear factor-κB signaling. Int. J. Nanomed. 2015, 20, 1463–1477. [Google Scholar] [CrossRef] [PubMed]
- Krętowski, R.; Stypułkowska, A.; Cechowska-Pasko, M. Efficient apoptosis and necrosis induction by proteasome inhibitor: Bortezomib in the DLD-1 human colon cancer cell line. Mol. Cell. Biochem. 2015, 398, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Tokgun, O.; Demiray, A.; Bulent, K.; Akca, H. Silica nanoparticles can induce apoptosis via dead receptor and caspase 8 pathway on A549 cells. Adv. Food Sci. 2015, 2, 65–70. [Google Scholar]
- Fubini, B.; Hubbard, A. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) generation by silica in inflammation and fibrosis. Free Radic. Biol. Med. 2003, 34, 1507–1516. [Google Scholar] [CrossRef]
- Ahmad, J.; Ahamed, M.; Akhtar, M.J.; Alrokayan, S.A.; Siddiqui, M.A.; Musarrat, J.; Al-Khedhairy, A.A. Apoptosis induction by silica nanoparticles mediated through reactive oxygen species in human liver cell line HepG2. Toxicol. Appl. Pharmacol. 2012, 259, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M. Silica nanoparticles-induced cytotoxicity oxidative stress and apoptosis in cultured A431 and A549 cells. Hum. Exp. Toxicol. 2013, 32, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Joshi, G.N.; Knecht, D.A. Silica phagocytosis causes apoptosis and necrosis by different temporal and molecular pathways in alveolar macrophages. Apoptosis 2013, 18, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Corbalan, J.J.; Medina, C.; Jacoby, A.; Malinski, T.; Radomski, M.W. Amorphous silica nanoparticles trigger nitric oxide/peroxynitrite imbalance in human endothelial cells: Inflammatory and cytotoxic effects. Int. J. Nanomed. 2011, 6, 2821–2835. [Google Scholar]
- Bauer, A.T.; Strozyk, E.A.; Gorzelanny, C.; Malinski, T.; Radomski, M.W. Cytotoxicity of silica nanoparticles through exocytosis of von Willebrand factor and necrotic cell death in primary human endothelial cells. Biomaterials 2011, 32, 8385–8393. [Google Scholar] [CrossRef] [PubMed]
- Zabirnyk, O.; Yezhelyev, M.; Seleverstov, O. Nanoparticles as a novel class of autophagy activators. Autophagy 2007, 3, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Yu, Y.; Yu, Y.; Li, Y.; Wang, J.; Geng, W.; Jiang, L.; Li, Q.; Zhou, X.; Sun, Z. Silica nanoparticles induce autophagy and endothelial dysfunction via the PI3K/Akt/mTOR signaling pathway. Int. J. Nanomed. 2014, 5, 5131–5141. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Duan, J.; Yu, Y.; Li, Y.; Liu, X.; Zhou, X.; Ho, K.F.; Tian, L.; Sun, Z. Silica nanoparticles induce autophagy and autophagic cell death in HepG2 cells triggered by reactive oxygen species. J. Hazard. Mater. 2014, 15, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Yang, M.; Jing, L.; Wang, J.; Yu, Y.; Li, Y.; Duan, J.; Zhou, X.; Li, Y.; Sun, Z. Amorphous silica nanoparticles trigger vascular endothelial cell injury through apoptosis and autophagy via reactive oxygen species-mediated MAPK/Bcl-2 and PI3K/Akt/mTOR signaling. Int. J. Nanomed. 2016, 11, 5257–5276. [Google Scholar] [CrossRef] [PubMed]
- Ventresca, E.M.; Lecht, S.; Jakubowski, P.; Chiaverelli, R.A.; Weaver, M.; Del Valle, L.; Ettinger, K.; Gincberg, G.; Priel, A.; Braiman, A.; et al. Association of p75 (NTR) and α9β1 integrin modulates NGF dependent cellular responses. Cell Signal. 2015, 27, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar] [PubMed]
- Kusaczuk, M.; Krętowski, R.; Bartoszewicz, M.; Cechowska-Pasko, M. Phenylbutyrate—A pan-HDAC inhibitor—Suppresses proliferation of glioblastoma LN-229 cell line. Tumour Biol. 2016, 37, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Kusaczuk, M.; Krętowski, R.; Stypułkowska, A.; Cechowska-Pasko, M. Molecular and cellular effects of a novel hydroxamate-based HDAC inhibitor—Belinostat—In glioblastoma cell lines: A preliminary report. Investig. New Drugs 2016, 34, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Alirezaei, M.; Fox, H.S.; Flynn, C.T.; Moore, C.S.; Hebb, A.L.; Frausto, R.F.; Bhan, V.; Kiosses, W.B.; Whitton, J.L.; Robertson, G.S.; et al. Elevated ATG5 expression in autoimmune demyelination and multiple sclerosis. Autophagy 2009, 5, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, 2002–2007. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Amaralm, C.; Borges, M.; Melo, S.; da Silva, E.T.; Correia-da-Silva, G.; Teixeira, N. Apoptosis and Autophagy in Breast Cancer Cells following Exemestane Treatment. PLoS ONE 2012, 7, e42398. [Google Scholar]
- De Oliveira, L.F.; Bouchmella, K.; Goncalves Kde, A.; Bettini, J.; Kobarg, J.; Cardoso, M.B. Functionalized Silica Nanoparticles As an Alternative Platform for Targeted Drug-Delivery of Water Insoluble Drugs. Langmuir 2016, 32, 3217–3225. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Keller, A.A. Natural and engineered nano and colloidal transport: Role of zeta potential in prediction of particle deposition. Langmuir 2009, 25, 6856–6862. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Yuan, Y.; Liu, Ch.; Wu, Y.; Lu, X.; Qian, J. Differential cytotoxicity and particle action of hydroxyapatite nanoparticles in human cancer cells. Nanomedicine 2014, 9, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Yang, X.; Yin, Q.; Cai, K.; Wang, H.; Chaudhury, I.; Yao, C.; Zhou, Q.; Kwon, M.; Hartman, J.A.; et al. Investigating the optimal size of anticancer nanomedicine. Proc. Natl. Acad. Sci. USA 2014, 28, 15344–15349. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Hondow, N.S.; Krzemiński, L.; Brown, A.P.; Jeuken, L.J.; Routledge, M.N. Mechanism of cellular uptake of genotoxic silica nanoparticles. Part. Fibre Toxicol. 2012, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Xie, L.; Fang, C.-J.; Yang, H.; Wang, Y.-J.; Zhen, X.-Y.; Yan, C.-H.; Wang, Y.; Zhao, M.; Peng, S. Implications for blood-brain-barrier permeability in vitro oxidative stress and neurotoxicity potential induced by mesoporous silica nanoparticles: Effects of surface modification. RCS Adv. 2016, 6, 2800–2890. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, J.; Xu, J.; Sun, L.; Chen, M.; Lan, M. Nano-SiO2 induces apoptosis via activation of p53 and Bax mediated by oxidative stress in human hepatic cell line. Toxicol. In Vitro 2010, 24, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Kim, H.; An, S.S.; Maeng, E.H.; Kim, M.K.; Song, Y.J. In vitro cytotoxicity of SiO2 or ZnO nanoparticles with different sizes and surface charges on U373MG human glioblastoma cells. Int. J. Nanomed. 2014, 15, 235–241. [Google Scholar]
- Napierska, D.; Thomassen, L.C.; Lison, D.; Martens, J.A.; Hoet, P.H. The nanosilica hazard: Another variable entity. Part. Fibre Toxicol. 2010, 7, 30–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.; Huang, Y.W.; Zhou, X.D.; Ma, Z. In vitro toxicity of silica nanoparticles in human lung cancer cells. Toxicol. Appl. Pharmacol. 2006, 217, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Chang, K.L.; Hwang, D.F.; Kong, Y.L. In vitro cytotoxicitiy of silica nanoparticles at high concentrations strongly depends on the metabolic activity type of the cell line. Environ. Sci. Technol. 2007, 41, 2064–2068. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Qian, J.; Zhou, H.; Gan, Q.; Tang, W.; Lu, J.; Yuan, Y.; Liu, C. In vitro cytotoxicity and induction of apoptosis by silica nanoparticles in human HepG2 hepatoma cells. Int. J. Nanomed. 2011, 6, 1889–1901. [Google Scholar]
- Li, Y.; Monteiro-Riviere, N.A. Mechanisms of cell uptake inflammatory potential and protein corona effects with gold nanoparticles. Nanomedicine 2016, 24, 3185–3203. [Google Scholar] [CrossRef] [PubMed]
- Sikora, A.; Shard, A.G.; Minelli, C. Size and ζ-Potential Measurement of Silica Nanoparticles in Serum Using Tunable Resistive Pulse Sensing. Langmuir 2016, 32, 2216–2224. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef] [PubMed]
- Wittmaack, K. In search of the most relevant parameter for quantifying lung inflammatory response to nanoparticle exposure: Particle number surface area or what? Environ. Health Perspect. 2007, 115, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Mytych, J.; Wnuk, M. Nanoparticle Technology as a Double-Edged Sword: Cytotoxic Genotoxic and Epigenetic Effects on Living Cells. J. Biomater. Nanobiotechnol. 2013, 4, 53–63. [Google Scholar]
- Bellezza, I.; Scarpelli, P.; Pizzo, S.; Grottelli, S.; Costanzi, E.; Minelli, A. ROS-independent Nrf2 activation in prostate cancer. Oncotarget 2017. Available online: https://www.researchgate.net/publication/317381564 (accessed on 7 June 2017).
- Oguz, S.; Kanter, M.; Erboga, M.; Toydemir, T.; Sayhan, M.B.; Onur, H. Effects of Urtica dioica on oxidative stress proliferation and apoptosis after partial hepatectomy in rats. Toxicol. Ind. Health 2015, 31, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, Y.; Liu, X.; Jin, M.; Zhang, L.; Du, Z.; Guo, C.; Huang, P.; Sun, Z. Cytotoxicity and mitochondrial damage caused by silica nanoparticles. Toxicol. In Vitro 2011, 25, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Alhadlaq, H.A.; Ahmad, J.; Siddiqui, M.A.; Khan, S.T.; Musarrat, J.; Al-Khedhairy, A.A. Comparative cytotoxicity of dolomite nanoparticles in human larynx HEp2 and liver HepG2 cells. J. Appl. Toxicol. 2015, 35, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Sana, R.; Calder, V.; Calonge, M.; Lee, W.; Wheeler, L.A.; Stern, M.E. Mitochondrial permeability transition pore in inflammatory apoptosis of human conjunctival epithelial cells and T cells: Effect of cyclosporin A. Investig. Ophthalmol. Vis. Sci. 2013, 54, 4717–4733. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, H.; Wang, Z.; He, S.; Chen, S.; Liao, D.; Wang, L.; Yan, J.; Liu, W.; Lei, X.; et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012, 148, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Nowak, J.S.; Mehn, D.; Nativo, P.; García, C.P.; Gioria, S.; Ojea-Jiménez, I.; Gilliland, D.; Rossi, F. Silica nanoparticle uptake induces survival mechanism in A549 cells by the activation of autophagy but not apoptosis. Toxicol. Lett. 2014, 224, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Krętowski, R.; Borzym-Kluczyk, M.; Stypułkowska, A.; Cechowska-Pasko, M. Low glucose dependent decrease of apoptosis and induction of autophagy in breast cancer MCF-7 cells. Mol. Cell. Biochem. 2016, 417, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg-Lerner, A.; Bialik, S.; Simon, H.U.; Kimchi, A. Life and death partners: Apoptosis autophagy and the cross talk between them. Cell Death Differ. 2009, 16, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Gump, J.M.; Thorburn, A. Autophagy and apoptosis: What is the connection? Trends Cell Biol. 2011, 21, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Yoshida, T.; Tsujioka, M.; Arakawa, S. Autophagic cell death and cancer. Int. J. Mol. Sci. 2014, 2, 3145–3153. [Google Scholar] [CrossRef] [PubMed]







| Times (h) | Deionized Water (A) | Medium (DMEM with 10% FBS) (B) | ||||
|---|---|---|---|---|---|---|
| Zeta Potential (mV) | Diameter (nm) | Zeta Potential (mV) | Diameter (nm) | |||
| Fraction I | Fraction II | Fraction I | Fraction II | |||
| 0 | −32.6 | 60.14 (99.1%) | 329.5 (0.9%) | −8.30 | 133.1 (100%) | - |
| 12 | −33.3 | 61.01 (97.5%) | 269.2 (2.5%) | −8.24 | 140.0 (100%) | - |
| 24 | −35.1 | 62.05 (98.2%) | 270.1 (1.8%) | −8.11 | 129.9 (94.0%) | 673.0 (6.0%) |
| 48 | −35.0 | 65.93 (98.4%) | 321.6 (1.6%) | −8.96 | 146.0 (95.9%) | 893.0 (4.1%) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krętowski, R.; Kusaczuk, M.; Naumowicz, M.; Kotyńska, J.; Szynaka, B.; Cechowska-Pasko, M. The Effects of Silica Nanoparticles on Apoptosis and Autophagy of Glioblastoma Cell Lines. Nanomaterials 2017, 7, 230. https://doi.org/10.3390/nano7080230
Krętowski R, Kusaczuk M, Naumowicz M, Kotyńska J, Szynaka B, Cechowska-Pasko M. The Effects of Silica Nanoparticles on Apoptosis and Autophagy of Glioblastoma Cell Lines. Nanomaterials. 2017; 7(8):230. https://doi.org/10.3390/nano7080230
Chicago/Turabian StyleKrętowski, Rafał, Magdalena Kusaczuk, Monika Naumowicz, Joanna Kotyńska, Beata Szynaka, and Marzanna Cechowska-Pasko. 2017. "The Effects of Silica Nanoparticles on Apoptosis and Autophagy of Glioblastoma Cell Lines" Nanomaterials 7, no. 8: 230. https://doi.org/10.3390/nano7080230
APA StyleKrętowski, R., Kusaczuk, M., Naumowicz, M., Kotyńska, J., Szynaka, B., & Cechowska-Pasko, M. (2017). The Effects of Silica Nanoparticles on Apoptosis and Autophagy of Glioblastoma Cell Lines. Nanomaterials, 7(8), 230. https://doi.org/10.3390/nano7080230