Effects of Various Surfactants on the Dispersion of MWCNTs–OH in Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
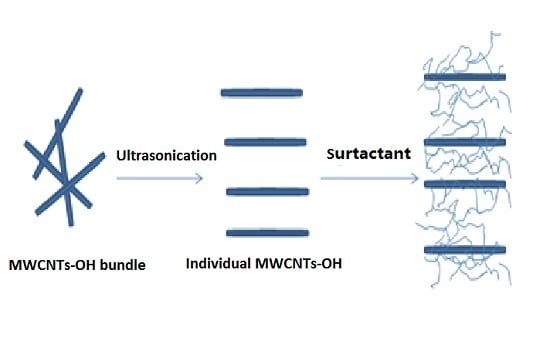
2.2. Preparation of MWCNTs–OH Dispersion with Various Surfactant
2.3. Characterization
2.3.1. Characterization of Raw MWCNTs–OH
2.3.2. Characterization of MWCNTs–OH Dispersion
3. Results and Discussion
3.1. Characterization of Raw MWCNTs–OH
3.2. Characterization of MWCNTs–OH Dispersions
3.2.1. Visual Observation
3.2.2. SEM Analyses
3.3. Comparison of Various MWCNTs–OH Dispersions Using UV–Vis Spectroscopy
3.4. Relationship between Dispersibility and Chemical Molecular Structure of Surfactant
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 350, 56–58. [Google Scholar] [CrossRef]
- Goze, C.; Vaccarini, L.; Henrard, L.; Bernier, P.; Hernandez, E.; Rubio, A. Elastic and mechanical properties of carbon nanotubes. Synth. Met. 1999, 103, 2500–2501. [Google Scholar] [CrossRef]
- Wilder, J.W.G.; Venema, L.C.; Rinzler, A.G.; Smalley, R.E.; Dekker, C. Electronic structure of atomically resolved carbon nanotubes. Nature 1998, 391, 59–62. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Charlier, J.-C.; Hernandez, E. Electronic, thermal and mechanical properties of carbon nanotubes. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 2004, 362, 2065–2098. [Google Scholar] [CrossRef] [PubMed]
- Kataura, H.; Kumazawa, Y.; Maniwa, Y.; Umezu, I.; Suzuki, S.; Ohtsuka, Y.; Achiba, Y. Optical properties of single-wall carbon nanotubes. Synth. Met. 1999, 103, 2555–2558. [Google Scholar] [CrossRef]
- Tang, X.; Bansaruntip, S.; Nakayama, N.; Yenilmez, E.; Chang, Y.I.; Wang, Q. Carbon nanotube DNA sensor and sensing mechanism. Nano Lett. 2006, 6, 1632–1636. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.L.; Mai, Y.W.; Zhou, X.P. Dispersion and alignment of carbon nanotubes in polymer matrix: A review. Mater. Sci. Eng. R Rep. 2005, 49, 89–112. [Google Scholar] [CrossRef]
- Koohsorkhi, J.; Abdi, Y.; Mohajerzadeh, S.; Hosseinzadegan, H.; Komijani, Y.; Soleimani, E.A. Fabrication of self-defined gated field emission devices on silicon substrates using PECVD-grown carbon nano-tubes. Carbon 2006, 44, 2797–2803. [Google Scholar] [CrossRef]
- Raymundo-Pinero, É.; Cazorla-Amoros, D.; Linares-Solano, A.; Delpeux, S.; Frackowiak, E.; Szostak, K.; Beguin, F. High surface area carbon nanotubes prepared by chemical activation. Carbon 2002, 40, 1597–1617. [Google Scholar] [CrossRef]
- Dai, H.; Hafner, J.H.; Rinzler, A.G.; Colbert, D.T.; Smalley, R.E. Nanotubes as nanoprobes in scanning probe microscopy. Nature 1996, 384, 147–150. [Google Scholar] [CrossRef]
- Vaisman, L.; Wagner, H.D.; Marom, G. The role of surfactants in dispersion of carbon nanotubes. Adv. Colloid Interface Sci. 2006, 128–130, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kro, M. A theoretical evaluation of the effects of carbon nanotube entanglement and bundling on the structural and mechanical properties of buckypaper. Carbon 2012, 50, 1793–1806. [Google Scholar] [CrossRef]
- Lourie, O.; Cox, D.M.; Wagner, H.D. Buckling and Collapse of Embedded Carbon Nanotubes. Phys. Rev. Lett. 1998, 81, 1638. [Google Scholar] [CrossRef]
- Girifalco, L.A.; Hodak, M.; Lee, R.S. Carbon nanotubes, buckyballs, ropes, and a universal graphitic potential. Phys. Rev. B Condens. Matter Mater. Phys. 2000, 62, 13104–13110. [Google Scholar] [CrossRef]
- Foundation, A.; Blaedel, W.J.; Wang, J.; Rochon, A.M.; Gesser, H.D.; Aubert, J.H.; Rand, P.B.; Arnold, C.; Clough, L.R.; Clough, R.L.; et al. Mechanical Damage of Carbon Nanotubes by Ultrasound. Carbon 1996, 34, 814–816. [Google Scholar]
- Papp, I.Z.; Kozma, G. Experimental validation of the Burgio e Rojac model of planetary ball milling by the length control of multiwall carbon nanotubes. Carbon 2016, 105, 615–621. [Google Scholar] [CrossRef]
- Konsta-Gdoutos, M.S.; Metaxa, Z.S.; Shah, S.P. Highly dispersed carbon nanotube reinforced cement based materials. Cem. Concr. Res. 2010, 40, 1052–1059. [Google Scholar] [CrossRef]
- Hilding, J.; Grulke, E.A.; George Zhang, Z.; Lockwood, F. Dispersion of Carbon Nanotubes in Liquids. J. Dispers. Sci. Technol. 2003, 24, 1–41. [Google Scholar] [CrossRef]
- Premkumar, T.; Mezzenga, R.; Geckeler, K.E. Carbon nanotubes in the liquid phase: Addressing the issue of dispersion. Small 2012, 8, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, W.; Ho, D.L.; Winey, K.I.; Fischer, J.E.; Glinka, C.J.; Hobbie, E.K. Dispersing single-walled carbon nanotubes with surfactants: A small angle neutron scattering study. Nano Lett. 2004, 4, 1789–1793. [Google Scholar] [CrossRef]
- Yu, J.; Grossiord, N.; Koning, C.E.; Loos, J. Controlling the dispersion of multi-wall carbon nanotubes in aqueous surfactant solution. Carbon 2007, 45, 618–623. [Google Scholar] [CrossRef]
- Whitsitt, E.A.; Barron, A.R. Silica coated single walled carbon nanotubes. Nano Lett. 2003, 3, 775–778. [Google Scholar] [CrossRef]
- Hough, L.A.; Islam, M.F.; Hammouda, B.; Yodh, A.G.; Heiney, P.A. Structure of semidilute single-wall carbon nanotube suspensions and gels. Am. Chem. Soc. 2006, 6, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Chen, S.J.; Korayem, A.H.; Collins, F.; Wang, C.M.; Duan, W.H. Effect of ultrasonication energy on engineering properties of carbon nanotube reinforced cement pastes. Carbon 2015, 85, 212–220. [Google Scholar] [CrossRef]
- Bai, Y.; Park, I.S.; Lee, S.J.; Bae, T.S.; Watari, F.; Uo, M.; Lee, M.H. Aqueous dispersion of surfactant-modified multiwalled carbon nanotubes and their application as an antibacterial agent. Carbon 2011, 49, 3663–3671. [Google Scholar] [CrossRef]
- Kumar, P.; Bohidar, H.B. Aqueous dispersion stability of multi-carbon nanoparticles in anionic, cationic, neutral, bile salt and pulmonary surfactant solutions. Colloids Surf. A Physicochem. Eng. Asp. 2010, 361, 13–24. [Google Scholar] [CrossRef]
- Pajootan, E.; Arami, M. Structural and electrochemical characterization of carbon electrode modified by multi-walled carbon nanotubes and surfactant. Electrochim. Acta 2013, 112, 505–514. [Google Scholar] [CrossRef]
- Xie, X.; Gao, L.; Sun, J. Thermodynamic study on aniline adsorption on chemical modified multi-walled carbon nanotubes. Colloids Surf. A Physicochem. Eng. Asp. 2007, 308, 54–59. [Google Scholar] [CrossRef]
- Hsin, Y.L.; Lai, J.Y.; Hwang, K.C.; Lo, S.C.; Chen, F.R.; Kai, J.J. Rapid surface functionalization of iron-filled multi-walled carbon nanotubes. Carbon 2006, 44, 3328–3335. [Google Scholar] [CrossRef]
- Jiang, L.; Gao, L.; Sun, J. Production of aqueous colloidal dispersions of carbon nanotubes. J. Colloid Interface Sci. 2003, 260, 89–94. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, H.; Qing, Q.; Yang, Y.; Li, Q.; Liu, Z.; Guo, X.; Du, Z. Effect of chemical oxidation on the structure of single-walled carbon nanotubes. J. Phys. Chem. B 2003, 107, 3712–3718. [Google Scholar] [CrossRef]
- Kim, U.J.; Furtado, C.A.; Liu, X.; Chen, G.; Eklund, P.C. Raman and IR spectroscopy of chemically processed single-walled carbon nanotubes. J. Am. Chem. Soc. 2005, 127, 15437–15445. [Google Scholar] [CrossRef] [PubMed]
- Mawhinney, D.B.; Naumenko, V.; Kuznetsova, A.; Yates, J.T.; Liu, J.; Smalley, R.E. Infrared spectral evidence for the etching of carbon nanotubes: Ozone oxidation at 298 K. J. Am. Chem. Soc. 2000, 122, 2383–2384. [Google Scholar] [CrossRef]
- Uddin, M.E.; Kuila, T.; Nayak, G.C.; Kim, N.H.; Ku, B.C.; Lee, J.H. Effects of various surfactants on the dispersion stability and electrical conductivity of surface modified graphene. J. Alloys Compd. 2013, 562, 134–142. [Google Scholar] [CrossRef]
- Chen, L.; Xie, H.; Li, Y.; Yu, W. Applications of cationic gemini surfactant in preparing multi-walled carbon nanotube contained nanofluids. Colloids Surf. A Physicochem. Eng. Asp. 2008, 330, 176–179. [Google Scholar] [CrossRef]
- Das, G.; Bettotti, P.; Ferraioli, L.; Raj, R.; Mariotto, G.; Pavesi, L.; Sorarù, G.D. Study of the pyrolysis process of an hybrid CH3SiO1.5 gel into a SiCO glass. Vib. Spectrosc. 2007, 45, 61–68. [Google Scholar] [CrossRef]
- Movia, D.; Del Canto, E.; Giordani, S. Purified and oxidized single-walled carbon nanotubes as robust near-IR fluorescent probes for molecular imaging. J. Phys. Chem. 2010, 114, 18407–18413. [Google Scholar] [CrossRef]
- Rance, G.A.; Marsh, D.H.; Nicholas, R.J.; Khlobystov, A.N. UV–Vis absorption spectroscopy of carbon nanotubes: Relationship between the π–electron plasmon and nanotube diameter. Chem. Phys. Lett. 2010, 493, 19–23. [Google Scholar] [CrossRef]
- Voisin, C.; Cassabois, G.; Roussignol, P.; Jost, O.; Goux-Capes, L.; Voisin, C.; Cassabois, G.; Delalande, C. Ultrafast carrier dynamics in single-wall carbon nanotubes. Phys. Rev. Lett. Am. Phys. Soc. 2003, 90, 057404. [Google Scholar]
- Rausch, J.; Zhuang, R.C.; Mäder, E. Surfactant assisted dispersion of functionalized multi-walled carbon nanotubes in aqueous media. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1038–1046. [Google Scholar] [CrossRef]
- Li, Q.; Church, J.S.; Kafi, A.; Naebe, M.; Fox, B.L. An improved understanding of the dispersion of multi-walled carbon nanotubes in non-aqueous solvents. J. Nanopart. Res. 2014, 16. [Google Scholar] [CrossRef]
- Asakura, S.; Oosawa, F. On interaction between two bodies immersed in a solution of macromolecules. J. Chem. Phys. 1954, 22, 1255–1256. [Google Scholar] [CrossRef]
- Bonard, J.; Stora, T.; Salvetat, J.; Maier, F.; Stockli, T.; Duschl, C.; Forro, L.; De Heer, W.A.; Andre, C. Purification and size-selection of carbon nanotubes. Adv. Mater. 1997, 9, 827–831. [Google Scholar] [CrossRef]
- Blanch, A.J.; Lenehan, C.E.; Quinton, J.S. Optimizing surfactant concentrations for dispersion of single-walled carbon nanotubes in aqueous solution. J. Phys. Chem. 2010, 114, 9805–9811. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.; Kaushal, R.; Tripathi, S.K.; Sharma, A.L.; Kaur, I.; Bharadwaj, L.M. Comparative study of carbon nanotube dispersion using surfactants. J. Colloid Interface Sci. 2008, 328, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.F.; Rojas, E.; Bergey, D.M.; Johnson, A.T.; Yodh, A.G. High weight fraction surfactant solubilization of single-wall carbon nanotubes in water. Nano Lett. 2003, 3, 269–273. [Google Scholar] [CrossRef]
- Chen, J.; Chen, W.; Zhu, D. Adsorption of nonionic aromatic compounds to single-walled carbon nanotubes: Effects of aqueous solution chemistry. Environ. Sci. Technol. 2008, 42, 7225–7230. [Google Scholar] [CrossRef] [PubMed]
- Cyr, D.M.; Venkataraman, B.; Flynn, G.W.; Black, A.; Whitesides, G.M. Functional group identification in scanning tunneling microscopy of molecular adsorbates. J. Phys. Chem. 1996, 100, 13747–13759. [Google Scholar] [CrossRef]
- Tan, Y.; Resasco, D.E. Dispersion of single-walled carbon nanotubes of narrow diameter distribution. J. Phys. Chem. B 2005, 109, 14454–14460. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Lin, D.; Wu, F.; Wang, Z.; Xing, B. Adsorption of Triton X-series surfactants and its role in stabilizing multi-walled carbon nanotube suspensions. Chemosphere 2010, 79, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.J.C.; Boccaccini, A.R.; Shaffer, M.S.P. Multi-walled carbon nanotube coatings using Electrophoretic Deposition (EPD). J. Am. Ceram. Soc. 2005, 88, 980–982. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, F.; Lin, D.; Xing, B. Clay minerals affect the stability of surfactant-facilitated carbon nanotube suspensions. Environ. Sci. Technol. 2008, 42, 6869–6875. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Stoffelbach, F.; Rieger, J.; Regnaud, L.; Vichot, A.; Bresson, B.; Lequeux, N. A new class of organosilane-modified polycarboxylate superplasticizers with low sulfate sensitivity. Cem. Concr. Res. 2012, 42, 166–172. [Google Scholar] [CrossRef]








| Type | OD | –OH Content | Length | Purity | Ash | SSA | EC |
|---|---|---|---|---|---|---|---|
| MWCNTs–OH | >50 nm | 0.76% | 20 μm | >90 wt % | <6 wt % | >40 m2/g | >102 s/cm |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.; Yan, X.; Monasterio, M.; Xing, F. Effects of Various Surfactants on the Dispersion of MWCNTs–OH in Aqueous Solution. Nanomaterials 2017, 7, 262. https://doi.org/10.3390/nano7090262
Cui H, Yan X, Monasterio M, Xing F. Effects of Various Surfactants on the Dispersion of MWCNTs–OH in Aqueous Solution. Nanomaterials. 2017; 7(9):262. https://doi.org/10.3390/nano7090262
Chicago/Turabian StyleCui, Hongzhi, Xiantong Yan, Manuel Monasterio, and Feng Xing. 2017. "Effects of Various Surfactants on the Dispersion of MWCNTs–OH in Aqueous Solution" Nanomaterials 7, no. 9: 262. https://doi.org/10.3390/nano7090262
APA StyleCui, H., Yan, X., Monasterio, M., & Xing, F. (2017). Effects of Various Surfactants on the Dispersion of MWCNTs–OH in Aqueous Solution. Nanomaterials, 7(9), 262. https://doi.org/10.3390/nano7090262