Light Trapping Effect in Perovskite Solar Cells by the Addition of Ag Nanoparticles, Using Textured Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Device Fabrication
2.3. Film and Device Characterization
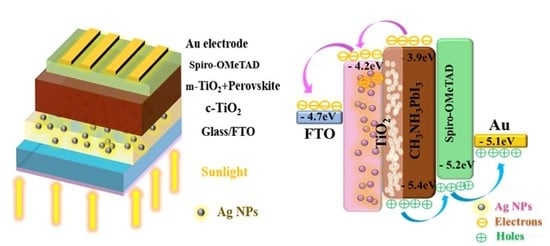
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chen, Q.; De Marco, N.; Yang, Y.; Song, T.-B.; Chen, C.-C.; Zhao, H.; Hong, Z.; Zhou, H.; Yang, Y. Under the spotlight: The organic-inorganic hybrid halide perovskite for optoelectronic applications. Nano Today 2015, 10, 355–396. [Google Scholar] [CrossRef]
- Ogomi, Y.; Morita, A.; Tsukamoto, S.; Saitho, T.; Fujikawa, N.; Shen, Q.; Toyoda, T.; Yoshino, K.; Pandey, S.S.; Ma, T.; et al. CH3NH3SnxPb(1−x)I3 Perovskite Solar Cells Covering up to 1060 nm. J. Phys. Chem. Lett. 2014, 5, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.H.; Im, S.H.; Heo, J.H.; Mandal, T.N.; Seok, S.I. Chemical management for colorful, efficient, and stable inorganic-organic hybrid nanostructured solar cells. Nano Lett. 2013, 13, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Ponseca, C.S., Jr.; Savenije, T.J.; Abdellah, M.; Zheng, K.; Yartsev, A.; Pascher, T.; Harlang, T.; Chabera, P.; Pullerits, T.; Stepanov, A.; et al. Organometal halide perovskite solar cell materials rationalized: Ultrafast charge generation, high and microsecond-long balanced mobilities, and slow recombination. J. Am. Chem. Soc. 2014, 136, 5189–5192. [Google Scholar] [CrossRef] [PubMed]
- Oga, H.; Saeki, A.; Ogomi, Y.; Hayase, S.; Seki, S. Improved understanding of the electronic and energetic landscapes of perovskite solar cells: High local charge carrier mobility, reduced recombination, and extremely shallow traps. J. Am. Chem. Soc. 2014, 136, 13818–13825. [Google Scholar] [CrossRef] [PubMed]
- Burschka, J.; Pellet, N.; Moon, S.J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Gratzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.Y.; Tsai, H.H.; Asadpour, R.; Blancon, J.C.; Neukirch, A.J.; Gupta, G.; Crochet, J.J.; Chhowalla, M.; Tretiak, S.; Alam, M.A.; et al. High-efficiency solution-processed perovskite solar cells with millimeter-scale grains. Science 2015, 347, 522–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, J.; Meng, L.; Song, T.B.; Guo, T.F.; Yang, Y.M.; Chang, W.H.; Hong, Z.; Chen, H.; Zhou, H.; Chen, Q.; et al. Improved air stability of perovskite solar cells via solution-processed metal oxide transport layers. Nat. Nanotechnol. 2016, 11, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Dar, M.I.; Hinderhofer, A.; Pellet, N.; Schreiber, F.; Zakeeruddin, S.M.; Grätzel, M. Perovskite solar cells with CuSCN hole extraction layers yield stabilized efficiencies greater than 20%. Science 2017, 358, 768–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Q.; Zhang, L.; Wang, H.; Yang, X.; Meng, J.; Liu, H.; Yin, Z.; Wu, J.; Zhang, X.; You, J. Enhanced electron extraction using SnO2 for high-efficiency planar-structure HC(NH2)2PbI3-based perovskite solar cells. Nat. Energy 2016, 2, 16177. [Google Scholar] [CrossRef]
- Sai, H.; Kanamori, Y.; Arafune, K.; Ohshita, Y.; Yamaguchi, M. Light trapping effect of submicron surface textures in crystalline Si solar cells. Prog. Photovolt. Res. Appl. 2007, 15, 415–423. [Google Scholar] [CrossRef]
- Eyderman, S.; Deinega, A.; John, S. Near perfect solar absorption in ultra-thin-film GaAs photonic crystals. J. Mater. Chem. A 2014, 2, 761–769. [Google Scholar] [CrossRef]
- Wang, D.H.; Seifter, J.; Park, J.H.; Choi, D.G.; Heeger, A.J. Efficiency increase in flexible bulk heterojunction solar cells with a nano-patterned indium zinc oxide anode. Adv. Energy Mater. 2012, 2, 1319–1322. [Google Scholar] [CrossRef]
- Luque, A.; Hegedus, S. Handbook of Photovoltaic Science and Engineering; Wiley: Chichester, UK, 2011. [Google Scholar]
- Yablonovitch, E. Statistical ray optics. J. Opt. Soc. Am. 1982, 72, 899–907. [Google Scholar] [CrossRef]
- Müller, J.; Rech, B.; Springer, J.; Vanecek, M. TCO and light trapping in silicon thin film solar cells. Sol. Energy 2004, 77, 917–930. [Google Scholar] [CrossRef]
- Lien, S.Y. Characterization and optimization of ITO thin films for application in heterojunction silicon solar cells. Thin Solid Films 2010, 518, S10–S13. [Google Scholar] [CrossRef]
- Soldera, M.; Taretto, K. Combining Thickness reduction and light trapping for potential efficiency improvements in perovskite solar cells. Phys. Status Solidi (A) 2018, 215, 1700906. [Google Scholar] [CrossRef]
- Li, G.; Shrotriya, V.; Huang, J.; Yao, Y.; Moriarty, T.; Emery, K.; Yang, Y. High-efficiency solution processable polymer photovoltaic cells by self-organization of polymer blends. Nat. Mater. 2005, 4, 864–868. [Google Scholar] [CrossRef]
- Pillai, S.A.; Green, M.A. Plasmonics for photovoltaic applications. Sol. Energy Mater. Sol. Cells 2010, 94, 1481–1486. [Google Scholar] [CrossRef]
- Atwater, H.A.; Polman, A. Plasmonics for improved photovoltaic devices. Nat. Mater. 2010, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Pan, X.; Ma, Y.; Li, Y.; Zheng, L.; Zhang, D.; Liu, F. Plasmonic-enhanced perovskite solar cells using alloy popcorn nanoparticles. RSC Adv. 2015, 5, 11175–11179. [Google Scholar] [CrossRef]
- Mali, S.S.; Shim, C.S.; Kim, H.; Patil, P.S.; Hong, C.K. In situ processed gold nanoparticle-embedded TiO2 nanofibers enabling plasmonic perovskite solar cells to exceed 14% conversion efficiency. Nanoscale 2016, 8, 2664–2677. [Google Scholar] [CrossRef] [PubMed]
- Fu, N.; Bao, Z.Y.; Zhang, Y.L.; Zhang, G.; Ke, S.; Lin, P.; Dai, J.; Huang, H.; Lei, D.Y. Panchromatic thin perovskite solar cells with broadband plasmonic absorption enhancement and efficient light scattering management by Au@ Ag core-shell nanocuboids. Nano Energy 2017, 41, 654–664. [Google Scholar] [CrossRef]
- Wang, B.; Zhu, X.Y.; Li, S.H.; Chen, M.W.; Lu, H.F.; Yang, Y.P. Ag@SiO2 core-shell nanoparticles embedded in a TiO2 mesoporous layer substantially improve the performance of perovskite solar cells. Nanomaterials 2018, 8, 701. [Google Scholar] [CrossRef] [PubMed]
- Erwin, W.R.; Zarick, H.F.; Talbert, E.M.; Bardhan, R. Light trapping in mesoporous solar cells with plasmonic nanostructures. Energy Environ. Sci. 2016, 9, 1577–1601. [Google Scholar] [CrossRef] [Green Version]
- Maier, S.A. Plasmonics: Fundamentals and Applications; Springer: New York, NY, USA, 2007. [Google Scholar]
- Marchioro, A.; Teuscher, J.; Friedrich, D.; Kunst, M.; Van De Krol, R.; Moehl, T.; Moser, J.E. Unravelling the mechanism of photoinduced charge transfer processes in lead iodide perovskite solar cells. Nat. Photonics 2014, 8, 250. [Google Scholar] [CrossRef]
- Zhong, D.; Cai, B.; Wang, X.; Yang, Z.; Xing, Y.; Miao, S.; Li, C. Synthesis of oriented TiO2 nanocones with fast charge transfer for perovskite solar cells. Nano Energy 2015, 11, 409–418. [Google Scholar] [CrossRef]
- Adhyaksa, G.W.P.; Baek, S.W.; Lee, G.I.; Lee, D.K.; Lee, J.Y.; Kang, J.K. Coupled near and far-field scattering in silver nanoparticles for high-efficiency, stable, and thin plasmonic dye-sensitized solar cells. ChemSusChem 2014, 7, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Beebe, J.M.; Engelkes, V.B.; Miller, L.L.; Frisbie, C.D. Contact resistance in metal- molecule- metal junctions based on aliphatic SAMs: Effects of surface linker and metal work function. J. Am. Chem. Soc. 2002, 124, 11268–11269. [Google Scholar] [CrossRef] [PubMed]
- Nourolahi, H.; Behjat, A.; Zarch, S.H.; Bolorizadeh, M.A. Silver nanoparticle plasmonic effects on hole-transport material-free mesoporous heterojunction perovskite solar cells. Sol. Energy 2016, 139, 475–483. [Google Scholar] [CrossRef]
- Hergert, W.; Wriedt, T. (Eds.) The Mie Theory: Basics and Applications; Springer: New York, NY, USA, 2012. [Google Scholar]
- Saliba, M.; Zhang, W.; Burlakov, V.M.; Stranks, S.D.; Sun, Y.; Ball, J.M.; Snaith, H.J. Plasmonic-induced photon recycling in metal halide perovskite solar cells. Adv. Funct. Mater. 2015, 25, 5038–5046. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, P.; Wei, L.; Liu, Z.; Fang, X.; Liu, X.; Mai, Y. Efficient charge-transport in hybrid lead iodide perovskite solar cells. Dalton Trans. 2015, 44, 16914–16922. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wu, Y.; Lu, Y.; Sun, Y.; Zhang, S.; Zhang, J.; Zhang, W.; Yuan, N.; Ding, J. Annealing-free perovskite films based on solvent engineering for efficient solar cells. J. Mater. Chem. C 2017, 5, 842–847. [Google Scholar] [CrossRef]









| Samples | Voc (V) | Jsc(mA/cm2) | FF | PCE (%) |
|---|---|---|---|---|
| Pristine | 0.91 | 20.03 | 0.51 | 9.29 |
| S-5 wt.% | 0.90 | 20.21 | 0.50 | 9.13 |
| S-6.7 wt.% | 0.92 | 21.03 | 0.51 | 9.72 |
| S-10 wt.% | 0.94 | 22.08 | 0.59 | 12.23 |
| S-20 wt.% | 0.97 | 21.41 | 0.49 | 10.18 |
| S-33.3 wt.% | 0.95 | 22.15 | 0.52 | 10.93 |
| T-10 wt.% | 1.02 | 22.89 | 0.60 | 14.01 |
| Samples | τ1 (ns) | A1 | τ2 (ns) | A2 | ket (109 s−1) | (ns) |
|---|---|---|---|---|---|---|
| Pristine | 0.486 | 0.0396 | 4.306 | 0.0135 | 1.825 | 3.356 |
| S-5 wt.% | 0.440 | 0.0638 | 4.472 | 0.0067 | 2.049 | 2.522 |
| S-10 wt.% | 0.423 | 0.0587 | 4.290 | 0.0084 | 2.131 | 2.712 |
| S-20 wt.% | 0.491 | 0.0524 | 4.493 | 0.0087 | 1.814 | 2.904 |
| T-10 wt.% | 0.464 | 0.0627 | 4.459 | 0.0067 | 1.931 | 2.488 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, J.; Hao, H.; Li, J.; Shi, L.; Zhong, T.; Zhang, C.; Dong, J.; Xing, J.; Liu, H.; Zhang, Z. Light Trapping Effect in Perovskite Solar Cells by the Addition of Ag Nanoparticles, Using Textured Substrates. Nanomaterials 2018, 8, 815. https://doi.org/10.3390/nano8100815
Hao J, Hao H, Li J, Shi L, Zhong T, Zhang C, Dong J, Xing J, Liu H, Zhang Z. Light Trapping Effect in Perovskite Solar Cells by the Addition of Ag Nanoparticles, Using Textured Substrates. Nanomaterials. 2018; 8(10):815. https://doi.org/10.3390/nano8100815
Chicago/Turabian StyleHao, Jiabin, Huiying Hao, Jianfeng Li, Lei Shi, Tingting Zhong, Chen Zhang, Jingjing Dong, Jie Xing, Hao Liu, and Zili Zhang. 2018. "Light Trapping Effect in Perovskite Solar Cells by the Addition of Ag Nanoparticles, Using Textured Substrates" Nanomaterials 8, no. 10: 815. https://doi.org/10.3390/nano8100815
APA StyleHao, J., Hao, H., Li, J., Shi, L., Zhong, T., Zhang, C., Dong, J., Xing, J., Liu, H., & Zhang, Z. (2018). Light Trapping Effect in Perovskite Solar Cells by the Addition of Ag Nanoparticles, Using Textured Substrates. Nanomaterials, 8(10), 815. https://doi.org/10.3390/nano8100815