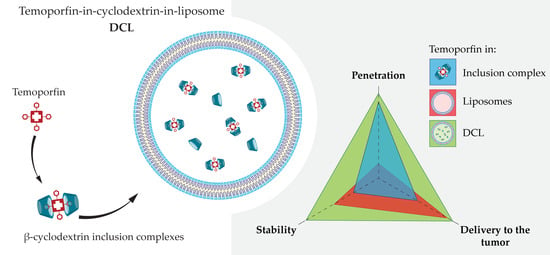
Temoporfin-in-Cyclodextrin-in-Liposome—A New Approach for Anticancer Drug Delivery: The Optimization of Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Drug-in-Cyclodextrin-in-Liposome Vesicles Preparation
2.2.1. Preparation of Inclusion Complexes
2.2.2. Thin Film Hydration
2.2.3. Purification of DCLs
2.3. Characterization of Liposomes
2.3.1. Determination of Encapsulation Efficiency (EE)
2.3.2. Photon Correlation Spectroscopy (PCS)
2.3.3. Atomic Force Microscopy (AFM)
2.3.4. Spectroscopic Measurements
2.4. Monolayer and Spheroid Cell Cultures
2.4.1. Culture Conditions
2.4.2. Generation of Spheroids
2.4.3. Imaging of mTHPC Distribution
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of mTHPC-DCLs
3.1.1. Preparation of mTHPC-DCLs
3.1.2. Size and Zeta Potential
3.1.3. mTHPC Localization in DCLs
3.1.4. Storage Stability
3.2. mTHPC Delivery to the Tumor Cells In Vitro
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marchal, S.; Hor, A.E.; Millard, M.; Gillon, V.; Bezdetnaya, L. Anticancer Drug Delivery: An Update on Clinically Applied Nanotherapeutics. Drugs 2015, 75, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, R.; Gan, Y.Y.; Soo, K.C.; Olivo, M. The effect of photodynamic therapy on tumor angiogenesis. Cell. Mol. Life Sci. 2009, 66, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Mroz, P.; Hamblin, M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer 2006, 6, 535–545. [Google Scholar] [CrossRef] [Green Version]
- West, C.M.; Moore, J.V. Mechanisms behind the resistance of spheroids to photodynamic treatment: A flow cytometry study. Photochem. Photobiol. 1992, 55, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Senge, M.O.; Brandt, J.C. Temoporfin (Foscan®, 5,10,15,20-tetra(m-hydroxyphenyl)chlorin)—A second-generation photosensitizer. Photochem. Photobiol. 2011, 87, 1240–1296. [Google Scholar] [CrossRef] [PubMed]
- Senge, M.O. Mthpc—A drug on its way from second to third generation photosensitizer? Photodiagnosis Photodyn. Ther. 2012, 9, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Yankovsky, I.; Bastien, E.; Yakavets, I.; Khludeyev, I.; Lassalle, H.-P.; Gräfe, S.; Bezdetnaya, L.; Zorin, V. Inclusion complexation with β-cyclodextrin derivatives alters photodynamic activity and biodistribution of meta-tetra(hydroxyphenyl)chlorin. Eur. J. Pharm. Sci. 2016, 91, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Yakavets, I.; Yankovsky, I.; Millard, M.; Lamy, L.; Lassalle, H.-P.; Wiehe, A.; Zorin, V.; Bezdetnaya, L. The alteration of temoporfin distribution in multicellular tumor spheroids by β-cyclodextrins. Int. J. Pharm. 2017, 529, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Yakavets, I.; Lassalle, H.-P.; Yankovsky, I.; Ingrosso, F.; Monari, A.; Bezdetnaya, L.; Zorin, V. Evaluation of temoporfin affinity to β-cyclodextrins assuming self-aggregation. J. Photochem. Photobiol. Chem. 2018, 367, 13–21. [Google Scholar] [CrossRef]
- McCormack, B.; Gregoriadis, G. Entrapment of cyclodextrin-drug complexes into liposomes: Potential advantages in drug delivery. J. Drug Target. 1994, 2, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Gharib, R.; Greige-Gerges, H.; Jraij, A.; Auezova, L.; Charcosset, C. Preparation of drug-in-cyclodextrin-in-liposomes at a large scale using a membrane contactor: Application to trans-anethole. Carbohydr. Polym. 2016, 154, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Dhule, S.S.; Penfornis, P.; Frazier, T.; Walker, R.; Feldman, J.; Tan, G.; He, J.; Alb, A.; John, V.; Pochampally, R. Curcumin-loaded γ-cyclodextrin liposomal nanoparticles as delivery vehicles for osteosarcoma. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 440–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnett, R.; Charlesworth, P.; Djelal, B.D.; Foley, S.; McGarvey, D.J.; Truscott, T.G. Photophysical properties of 5,10,15,20-tetrakis(m-hydroxyphenyl)porphyrin (m-THPP), 5,10,15,20-tetrakis(m-hydroxyphenyl)chlorin (m-THPC) and 5,10,15,20-tetrakis(m-hydroxyphenyl)bacteriochlorin (m-THPBC): A comparative study. J. Chem. Soc. Perkin Trans. 2 1999, 325–328. [Google Scholar] [CrossRef]
- Maestrelli, F.; González-Rodríguez, M.L.; Rabasco, A.M.; Mura, P. Preparation and characterisation of liposomes encapsulating ketoprofen–cyclodextrin complexes for transdermal drug delivery. Int. J. Pharm. 2005, 298, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Reshetov, V.; Kachatkou, D.; Shmigol, T.; Zorin, V.; D’Hallewin, M.-A.; Guillemin, F.; Bezdetnaya, L. Redistribution of meta-tetra(hydroxyphenyl)chlorin (m-THPC) from conventional and PEGylated liposomes to biological substrates. Photochem. Photobiol. Sci. 2011, 10, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Gharib, R.; Greige-Gerges, H.; Fourmentin, S.; Charcosset, C.; Auezova, L. Liposomes incorporating cyclodextrin-drug inclusion complexes: Current state of knowledge. Carbohydr. Polym. 2015, 129, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Lasch, J.; Weissig, V.; Brandl, M. Liposomes: A Practical Approach, 2nd ed.; Torchilin, V., Weissig, V., Eds.; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Marchal, S.; Fadloun, A.; Maugain, E.; D’Hallewin, M.-A.; Guillemin, F.; Bezdetnaya, L. Necrotic and apoptotic features of cell death in response to Foscan photosensitization of HT29 monolayer and multicell spheroids. Biochem. Pharmacol. 2005, 69, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-X.; Feng, S.-S.; Zheng, C.-H. A comparison between conventional liposome and drug-cyclodextrin complex in liposome system. Int. J. Pharm. 2016, 513, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Q.; Wang, X.; Zhang, W.; Lin, C.; Chen, F.; Yang, X.; Pan, W. Drug-in-cyclodextrin-in-liposomes: A novel drug delivery system for flurbiprofen. Int. J. Pharm. 2015, 492, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Arima, H.; Hagiwara, Y.; Hirayama, F.; Uekama, K. Enhancement of antitumor effect of doxorubicin by its complexation with gamma-cyclodextrin in pegylated liposomes. J. Drug Target. 2006, 14, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, W.-L.; Gu, W.; Lu, S.-S.; Chen, Z.-P.; Cai, B.-C.; Yang, X.-X. Drug-in-cyclodextrin-in-liposomes: A promising delivery system for hydrophobic drugs. Expert Opin. Drug Deliv. 2014, 11, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Reshetov, V.; Lassalle, H.-P.; François, A.; Dumas, D.; Hupont, S.; Gräfe, S.; Filipe, V.; Jiskoot, W.; Guillemin, F.; Zorin, V.; et al. Photodynamic therapy with conventional and PEGylated liposomal formulations of mTHPC (temoporfin): Comparison of treatment efficacy and distribution characteristics in vivo. Int. J. Nanomed. 2013, 8, 3817–3831. [Google Scholar] [CrossRef] [PubMed]
- Reshetov, V.; Zorin, V.; Siupa, A.; D’Hallewin, M.-A.; Guillemin, F.; Bezdetnaya, L. Interaction of liposomal formulations of meta-tetra(hydroxyphenyl)chlorin (temoporfin) with serum proteins: Protein binding and liposome destruction. Photochem. Photobiol. 2012, 88, 1256–1264. [Google Scholar] [CrossRef] [PubMed]
- Lyklema, J.; Fleer, G.J. Electrical contributions to the effect of macromolecules on colloid stability. Colloids Surf. 1987, 25, 357–368. [Google Scholar] [CrossRef]
- Hunter, R.J.; Midmore, B.R.; Zhang, H. Zeta Potential of Highly Charged Thin Double-Layer Systems. J. Colloid Interface Sci. 2001, 237, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.D.; Nir, S.; Papahadjopoulos, D. Quantitative analysis of liposome-cell interactions in vitro: Rate constants of binding and endocytosis with suspension and adherent J774 cells and human monocytes. Biochemistry 1993, 32, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Chonn, A.; Semple, S.C.; Cullis, P.R. Association of blood proteins with large unilamellar liposomes in vivo. Relation to circulation lifetimes. J. Biol. Chem. 1992, 267, 18759–18765. [Google Scholar] [PubMed]
- Szente, L.; Fenyvesi, É. Cyclodextrin-Lipid Complexes: Cavity Size Matters. Struct. Chem. 2017, 28, 479–492. [Google Scholar] [CrossRef]
- Yakavets, I.V.; Yankovsky, I.V.; Khludeyev, I.I.; Lassalle, H.P.; Bezdetnaya, L.N.; Zorin, V.P. Optical Methods for the Analysis of the Temoprofin Photosensitizer Distribution Between Serum Proteins and Methyl-β-Cyclodextrin Nanocarriers in Blood Serum. J. Appl. Spectrosc. 2018, 84, 1030–1036. [Google Scholar] [CrossRef]
- Yakavets, I.; Yankovsky, I.; Bezdetnaya, L.; Zorin, V. Soret band shape indicates mTHPC distribution between β-cyclodextrins and serum proteins. Dyes Pigments 2017, 137, 299–306. [Google Scholar] [CrossRef]
- Piel, G.; Piette, M.; Barillaro, V.; Castagne, D.; Evrard, B.; Delattre, L. Betamethasone-in-cyclodextrin-in-liposome: The effect of cyclodextrins on encapsulation efficiency and release kinetics. Int. J. Pharm. 2006, 312, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Puskás, I.; Barcza, L.; Szente, L.; Csempesz, F. Features of the Interaction between Cyclodextrins and Colloidal Liposomes. J. Incl. Phenom. Macrocycl. Chem. 2006, 54, 89–93. [Google Scholar] [CrossRef]
- Uekama, K.; Otagiri, M. Cyclodextrins in drug carrier systems. Crit. Rev. Ther. Drug Carr. Syst. 1987, 3, 1–40. [Google Scholar] [CrossRef]
- Fatouros, D.G.; Hatzidimitriou, K.; Antimisiaris, S.G. Liposomes encapsulating prednisolone and prednisolone–cyclodextrin complexes: Comparison of membrane integrity and drug release. Eur. J. Pharm. Sci. 2001, 13, 287–296. [Google Scholar] [CrossRef]
- Kiesslich, T.; Berlanda, J.; Plaetzer, K.; Krammer, B.; Berr, F. Comparative characterization of the efficiency and cellular pharmacokinetics of Foscan- and Foslip-based photodynamic treatment in human biliary tract cancer cell lines. Photochem. Photobiol. Sci. 2007, 6, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 2016, 6, 19103. [Google Scholar] [CrossRef] [PubMed]
- Millard, M.; Yakavets, I.; Zorin, V.; Kulmukhamedova, A.; Marchal, S.; Bezdetnaya, L. Drug Delivery to Solid Tumors: The Predictive Value of the Multicellular Tumor Spheroid Model for Nanomedicine Screening. Available online: https://www.dovepress.com/drug-delivery-to-solid-tumors-the--predictive-value-of-the-multicellul-peer-reviewed-article-IJN (accessed on 19 February 2018).
- Gaio, E.; Scheglmann, D.; Reddi, E.; Moret, F. Uptake and photo-toxicity of Foscan®, Foslip® and Fospeg® in multicellular tumor spheroids. J. Photochem. Photobiol. B 2016, 161, 244–252. [Google Scholar] [CrossRef] [PubMed]






| Formulation | CD | CD (mM) | mTHPC (mM) | Lipid (mM) |
|---|---|---|---|---|
| Empty liposomes | - | - | - | 26 |
| DCLs with Hp-β-CD 1 | ||||
| HDCL 1 | Hp-β-CD | 200 | 5 | 26 |
| HDCL 2 | 1.7 | |||
| HDCL 3 | 0.5 | |||
| DCLs with Me-β-CD 2 | ||||
| MDCL 1 | Me-β-CD | 20 | 5 | 26 |
| MDCL 2 | 1.7 | |||
| MDCL 3 | 0.5 | |||
| DCLs with TM-β-CD 3 | ||||
| TDCL 1 | TM-β-CD | 10 | 5 | 26 |
| TDCL 2 | 1.7 | |||
| TDCL 3 | 0.5 |
| Formulation | EE (%) | Size (nm) | Polydispersity Index | Zeta-Potential (mV) |
|---|---|---|---|---|
| Foslip® | >85 [16] | 113.6 ± 0.7 | 0.110 ± 0.015 | −34.4 ± 4.3 |
| DCLs with Hp-β-CD | ||||
| HDCL 1 | 7 | 137.7 ± 3.3 | 0.067 ± 0.030 | −38.1 ± 1.9 |
| HDCL 2 | 13 | 128.5 ± 0.3 | 0.037 ± 0.015 | −36.7 ± 0.8 |
| HDCL 3 | 17 | 125.7 ± 0.9 | 0.050 ± 0.004 | −37.3 ± 1.6 |
| DCLs with Me-β-CD | ||||
| MDCL 1 | 5 | 132.7 ± 0.8 | 0.045 ± 0.012 | −37.8 ± 1.8 |
| MDCL 2 | 7 | 141.0 ± 2.2 | 0.101 ± 0.019 | −39.0 ± 2.6 |
| MDCL 3 | 9 | 142.2 ± 0.8 | 0.073 ± 0.022 | −36.4 ± 0.9 |
| DCLs with TM-β-CD | ||||
| TDCL 1 | 7 | 135.9 ± 1.4 | 0.101 ± 0.034 | −38.1 ± 1.2 |
| TDCL 2 | 9 | 139.2 ± 0.9 | 0.065 ± 0.017 | −36.9 ± 1.5 |
| TDCL 3 | 14 | 130.6 ± 1.3 | 0.078 ± 0.026 | −37.3 ± 2.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakavets, I.; Lassalle, H.-P.; Scheglmann, D.; Wiehe, A.; Zorin, V.; Bezdetnaya, L. Temoporfin-in-Cyclodextrin-in-Liposome—A New Approach for Anticancer Drug Delivery: The Optimization of Composition. Nanomaterials 2018, 8, 847. https://doi.org/10.3390/nano8100847
Yakavets I, Lassalle H-P, Scheglmann D, Wiehe A, Zorin V, Bezdetnaya L. Temoporfin-in-Cyclodextrin-in-Liposome—A New Approach for Anticancer Drug Delivery: The Optimization of Composition. Nanomaterials. 2018; 8(10):847. https://doi.org/10.3390/nano8100847
Chicago/Turabian StyleYakavets, Ilya, Henri-Pierre Lassalle, Dietrich Scheglmann, Arno Wiehe, Vladimir Zorin, and Lina Bezdetnaya. 2018. "Temoporfin-in-Cyclodextrin-in-Liposome—A New Approach for Anticancer Drug Delivery: The Optimization of Composition" Nanomaterials 8, no. 10: 847. https://doi.org/10.3390/nano8100847
APA StyleYakavets, I., Lassalle, H. -P., Scheglmann, D., Wiehe, A., Zorin, V., & Bezdetnaya, L. (2018). Temoporfin-in-Cyclodextrin-in-Liposome—A New Approach for Anticancer Drug Delivery: The Optimization of Composition. Nanomaterials, 8(10), 847. https://doi.org/10.3390/nano8100847