Boron Nitride as a Novel Support for Highly Stable Palladium Nanocatalysts by Atomic Layer Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Atomic Layer Deposition of Boron Nitride
2.2. Atomic Layer Deposition of Palladium
2.3. Thermal Treatments
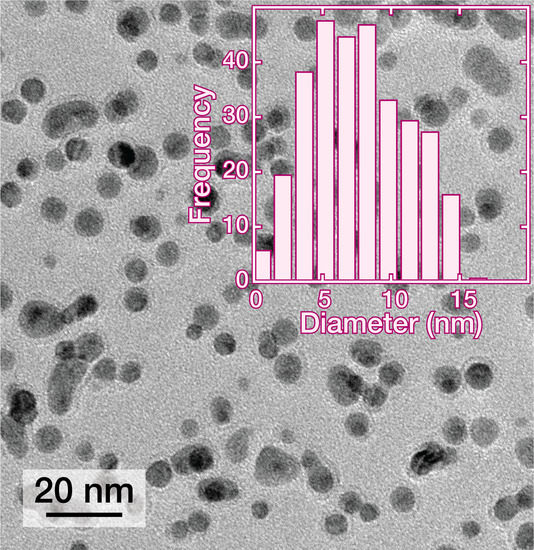
2.4. Transmission Electron Microscopy Imaging and Analysis of the Palladium NPs
2.5. Chemical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gallon, B.J.; Kojima, R.W.; Kaner, R.B.; Diaconescu, P.L. Palladium nanoparticles supported on Polyaniline nanofibers as a semi-heterogeneous catalyst in Water. Angew. Chem. Int. Ed. 2007, 46, 7251–7254. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.M.; Thomas, W.J. Principles and Practice of Heterogeneous Catalysis; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Kim, B.H.; Hackett, M.J.; Park, J.; Hyeon, T. Synthesis, characterization, and application of ultrasmall nanoparticles. Chem. Mater. 2013, 26, 59–71. [Google Scholar] [CrossRef]
- Campelo, J.M.; Luna, D.; Luque, R.; Marinas, J.M.; Romero, A.A. Sustainable preparation of supported metal nanoparticles and their applications in catalysis. Chem. Sus. Chem. 2009, 2, 18–45. [Google Scholar] [CrossRef] [PubMed]
- White, R.J.; Luque, R.; Budarin, V.L.; Clark, J.H.; Macquarrie, D.J. Supported metal nanoparticles on porous materials. Methods and applications. Chem. Soc. Rev. 2009, 38, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Q.; Wang, Y.; Wan, H. Size-dependent catalytic activity of supported palladium nanoparticles for aerobic oxidation of alcohols. Adv. Synth. Catal. 2008, 350, 453–464. [Google Scholar] [CrossRef]
- Xu, Z.; Xiao, F.-S.; Purnell, S.K.; Alexeev, O.; Kawi, S.; Deutsch, S.E.; Gates, B.C. Size-dependent catalytic activity of supported metal clusters. Nature 1994, 372, 346–348. [Google Scholar] [CrossRef]
- Shao, M.; Peles, A.; Shoemaker, K. Electrocatalysis on platinum nanoparticles: Particle size effect on oxygen reduction reaction activity. Nano Lett. 2011, 11, 3714–3719. [Google Scholar] [CrossRef] [PubMed]
- Rioux, R.M.; Song, H.; Grass, M.; Habas, S.; Niesz, K.; Hoefelmeyer, J.D.; Yang, P.; Somorjai, G.A. Monodisperse platinum nanoparticles of well-defined shape: Synthesis, characterization, catalytic properties and future prospects. Top. Catal. 2006, 39, 167–174. [Google Scholar] [CrossRef]
- Tauster, S.J.; Fung, S.C.; Garten, R.L. Strong metal-support interactions. Group 8 noble metals supported on titanium dioxide. J. Am. Chem. Soc. 1978, 100, 170–175. [Google Scholar] [CrossRef]
- Monyoncho, E.A.; Ntais, S.; Brazeau, N.; Wu, J.; Sun, C.; Baranova, E.A. Role of the metal-oxide support in the catalytic activity of Pd nanoparticles for ethanol electrooxidation in alkaline media. Chem Electro.Chem. 2016, 3, 218–227. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous catalyst deactivation and regeneration: A review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Sun, X.A.; Saha, M.S. Nanotubes, nanofibers and nanowires as supports for catalysts. In PEM Fuel Cell Electrocatalysts and Catalyst Layers; Zhang, J., Ed.; Springer: London, UK, 2008; pp. 655–714. [Google Scholar]
- Prasad, R.; Kennedy, L.A.; Ruckenstein, E. Catalytic combustion. Catal. Rev. Sci. Eng. 1984, 26, 1–58. [Google Scholar] [CrossRef]
- Bauer, J.E.; Occelli, M.L.; Williams, P.M.; McCaslin, P.C. Heterogeneous catalyst structure and function: Review and implications for the analysis of dissolved organic carbon and nitrogen in natural waters. Mar. Chem. 1993, 41, 75–89. [Google Scholar] [CrossRef]
- Bechelany, M.; Brioude, A.; Stadelmann, P.; Bernard, S.; Cornu, D.; Miele, P. Preparation of BN microtubes/nanotubes with a unique chemical process. J. Phys. Chem. C 2008, 112, 18325–18330. [Google Scholar] [CrossRef]
- Lipp, A.; Schwetz, K.A.; Hunold, K. Hexagonal boron nitride: Fabrication, properties and applications. J. Eur. Ceram. Soc. 1989, 5, 3–9. [Google Scholar] [CrossRef]
- Chen, Y.; Zou, J.; Campbell, S.J.; Le Caer, G. Boron nitride nanotubes: Pronounced resistance to oxidation. Appl. Phys. Lett. 2004, 84, 2430–2432. [Google Scholar] [CrossRef]
- Bernard, S.; Salles, V.; Li, J.; Brioude, A.; Bechelany, M.; Demirci, U.B.; Miele, P. High-yield synthesis of hollow boron nitride nano-polyhedrons. J. Mater. Chem. 2011, 21, 8694–8699. [Google Scholar] [CrossRef]
- Weng, Q.; Wang, X.; Zhi, C.; Bando, Y.; Golberg, D. Boron nitride porous microbelts for hydrogen storage. ACS Nano 2013, 7, 1558–1565. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, J.; Xu, X.; Zhang, X.; Xue, Y.; Mi, J.; Mo, Z.; Fan, Y.; Hu, L.; Yang, X. Porous boron nitride with a high surface area: Hydrogen storage and water treatment. Nanotechnology 2013, 24, 155603. [Google Scholar] [CrossRef] [PubMed]
- Postole, G.; Caldararu, M.; Ionescu, N.I.; Bonnetot, B.; Auroux, A.; Guimon, C. Boron nitride: A high potential support for combustion catalysts. Thermochim. Acta 2005, 434, 150–157. [Google Scholar] [CrossRef]
- Meyer, N.; Bekaert, K.; Pirson, D.; Devillers, M.; Hermans, S. Boron nitride as an alternative support of Pd catalysts for the selective oxidation of lactose. Catal. Commun. 2012, 29, 170–174. [Google Scholar] [CrossRef]
- Wu, J.C.S.; Chen, C.-Y.; Lin, S.D. Boron nitride supported Pt catalyst for selective hydrogenation. Catal. Lett. 2005, 102, 223–227. [Google Scholar] [CrossRef]
- Gao, L.; Fu, Q.; Wei, M.; Zhu, Y.; Liu, Q.; Crumlin, E.; Liu, Z.; Bao, X. Enhanced nickel-catalyzed methanation confined under hexagonal boron nitride shells. ACS Catal. 2016, 6, 6814–6822. [Google Scholar] [CrossRef]
- Schimmenti, R.; Cortese, R.; Duca, D.; Mavrikakis, M. Boron Nitride-supported Sub-nanometer Pd6 Clusters for Formic Acid Decomposition: A DFT Study. ChemCatChem. 2017, 9, 1610–1620. [Google Scholar] [CrossRef] [Green Version]
- Meyer, N.; Devillers, M.; Hermans, S. Boron nitride supported Pd catalysts for the hydrogenation of lactose. Catal. Today 2015, 241, 200–207. [Google Scholar] [CrossRef]
- Yabe, Y.; Sawama, Y.; Yamada, T.; Nagata, S.; Monguchi, Y.; Sajiki, H. Easily-controlled chemoselective hydrogenation by using palladium on boron nitride. ChemCatChem. 2013, 5, 2360–2366. [Google Scholar] [CrossRef]
- Zuo, L.-X.; Jiang, L.-P. Electrocatalysis of the oxygen reduction reaction and the formic acid oxidation reaction on BN/Pd composites prepared sonochemically. J. Electrochem. Soc. 2017, 164, H805–H811. [Google Scholar] [CrossRef]
- Ritala, M.; Kukli, K.; Rahtu, A.; Räisänen, P.I.; Leskelä, M.; Sajavaara, T.; Keinonen, J. Atomic layer deposition of oxide thin films with metal alkoxides as oxygen sources. Science 2000, 288, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Hämäläinen, J.; Ritala, M.; Leskelä, M. Atomic layer deposition of noble metals and their oxides. Chem. Mater. 2013, 26, 786–801. [Google Scholar] [CrossRef]
- Mårlid, B.; Ottosson, M.; Pettersson, U.; Larsson, K.; Carlsson, J.O. Atomic layer deposition of BN thin films. Thin Solid Films 2002, 402, 167–171. [Google Scholar] [CrossRef]
- Kim, H. Atomic layer deposition of metal and nitride thin films: Current research efforts and applications for semiconductor device processing. J. Vac. Sci. Technol. B Microelectron. Nano. Struct. Process. Meas. Phenom. 2003, 21, 2231–2261. [Google Scholar] [CrossRef]
- Weber, M.; Koonkaew, B.; Balme, S.; Utke, I.; Picaud, F.; Iatsunskyi, I.; Coy, E.; Miele, P.; Bechelany, M. Boron nitride nanoporous nembranes with high surface charge by atomic layer deposition. ACS Appl. Mater. Interfaces 2017, 9, 16669–16678. [Google Scholar] [CrossRef] [PubMed]
- Assaud, L.; Pitzschel, K.; Hanbücken, M.; Santinacci, L. Highly-conformal TiN thin films grown by thermal and plasma-enhanced atomic layer deposition. ECS J. Solid State Sci. Technol. 2014, 3, 253–258. [Google Scholar] [CrossRef]
- Aaltonen, T.; Ritala, M.; Tung, Y.-L.; Chi, Y.; Arstila, K.; Meinander, K.; Leskelä, M. Atomic layer deposition of noble metals: Exploration of the low limit of the deposition temperature. J. Mater. Res. 2004, 19, 3353–3358. [Google Scholar] [CrossRef]
- Weber, M.J.; Mackus, A.J.M.; Verheijen, M.A.; Longo, V.; Bol, A.A.; Kessels, W.M.M. Atomic layer deposition of high-purity palladium films from Pd(hfac)2 and H2 and O2 plasmas. J. Phys. Chem. C 2014, 118, 8702. [Google Scholar] [CrossRef]
- Leskelä, M.; Ritala, M. Atomic layer deposition (ALD): From precursors to thin film structures. Thin Solid Films 2002, 409, 138–146. [Google Scholar] [CrossRef]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Putkonen, M. Precursors for ALD Processes. In Atomic Layer Deposition of Nanostructured Materials; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 41–59. [Google Scholar]
- Zaera, F. The surface chemistry of thin film atomic layer deposition (ALD) processes for electronic device manufacturing. J. Mater. Chem. 2008, 18, 3521–3526. [Google Scholar] [CrossRef]
- Graniel, O.M.; Weber, S.; Balme, P.; Miele, M.B. Atomic layer deposition for biosensing applications. Biosens. Bioelectron. 2018, 122, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Van Delft, J.A.; Garcia-Alonso, D.; Kessels, W.M.M. Atomic layer deposition for photovoltaics: Applications and prospects for solar cell manufacturing. Semicond. Sci. Technol. 2012, 27, 74002. [Google Scholar] [CrossRef]
- Weber, M.A.; Julbe, A.; Ayral, P.; Miele, M.B. Atomic layer deposition for membranes: Basics, challenges and opportunities. Chem. Mater. 2018. [Google Scholar] [CrossRef]
- Marichy, C.; Bechelany, M.; Pinna, N. Atomic layer deposition of nanostructured materials for energy and environmental applications. Adv. Mater. 2012, 24, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Detavernier, C.; Dendooven, J.; Pulinthanathu Sree, S.; Ludwig, K.F.; Martens, J.A. Tailoring nanoporous materials by atomic layer deposition. Chem. Soc. Rev. 2011, 40, 5242–5253. [Google Scholar] [CrossRef] [PubMed]
- Van Bui, H.; Grillo, F.; van Ommen, J.R. Atomic and molecular layer deposition: Off the beaten track. Chem. Commun. 2017, 53, 45–71. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, J.D.; Weimer, A.W.; George, S.M. Atomic layer deposition of boron nitride using sequential exposures of BCl3 and NH3. Thin Solid Films 2002, 413, 16–25. [Google Scholar] [CrossRef]
- Olander, J.; Ottosson, L.M.; Heszler, P.; Carlsson, J.-O.; Larsson, K.M.E. Laser-assisted atomic layer deposition of boron nitride thin films. Chem. Vap. Depos. 2005, 11, 330–337. [Google Scholar] [CrossRef]
- Park, H.; Kim, T.K.; Cho, S.W.; Jang, H.S.; Lee, S.I.; Choi, S.-Y. Large-scale synthesis of uniform hexagonal boron nitride films by plasma-enhanced atomic layer deposition. Sci. Rep. 2017, 7, 40091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemmi, A.; Bernard, C.; Cun, H.; Roth, S.; Klöckner, M.; Weinl, M.; Gsell, S.; Schreck, M.; Osterwalder, J.; Greber, T. High quality single atomic layer deposition of hexagonal boron nitride on single crystalline Rh(111) four-inch wafers. Rev. Sci. Instrum. 2014, 85, 35101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprenger, J.K.; Sun, H.; Cavanagh, A.S.; Roshko, A.; Blanchard, P.T.; George, S.M. Electron-enhanced atomic layer deposition of boron nitride thin films at room temperature and 100 °C. J. Phys. Chem. C 2018, 122, 9455–9464. [Google Scholar] [CrossRef]
- Weber, M.; Iatsunskyi, I.; Coy, E.; Miele, P.; Cornu, D.; Bechelany, M. Novel and facile route for the synthesis of tunable boron nitride nanotubes combining atomic layer deposition and annealing processes for water purification. Adv. Mater. Interfaces 2018, 5, 18–56. [Google Scholar] [CrossRef]
- Hao, W.; Marichy, C.; Journet, C.; Brioude, A. A novel two-step ammonia-free atomic layer deposition approach for boron nitride. Chem. Nano. Mat. 2017, 3, 656–663. [Google Scholar] [CrossRef]
- Campbell, C.T. Ultrathin metal films and particles on oxide surfaces: Structural, electronic and chemisorptive properties. Surf. Sci. Rep. 1997, 27, 1–111. [Google Scholar] [CrossRef]
- Mackus, A.J.M.; Weber, M.J.; Thissen, N.F.W.; Garcia-Alonso, D.; Vervuurt, R.H.J.; Assali, S.; Bol, A.A.; Verheijen, M.A.; Kessels, W.M.M. Atomic layer deposition of Pd and Pt nanoparticles for catalysis: On the mechanisms of nanoparticle formation. Nanotechnology 2016, 27, 34001. [Google Scholar] [CrossRef] [PubMed]
- Elam, J.W.; Zinovev, A.V.V.; Pellin, M.J.; Comstock, D.J.; Hersam, M.C. Nucleation and growth of noble metals on oxide surfaces using atomic layer deposition. ECS Trans. 2007, 3, 271–278. [Google Scholar] [CrossRef]
- Leick, N.; Weber, J.W.; Mackus, A.J.M.; Weber, M.J.; Van de Sanden, M.C.M.; Kessels, W.M.M. In situ spectroscopic ellipsometry during atomic layer deposition of Pt, Ru and Pd. J. Phys. D Appl. Phys. 2016, 49, 115504. [Google Scholar] [CrossRef]
- O’Neill, B.J.; Jackson, D.H.K.; Lee, J.; Canlas, C.; Stair, P.C.; Marshall, C.L.; Elam, J.W.; Kuech, T.F.; Dumesic, J.A.; Huber, G.W. Catalyst design with atomic layer deposition. ACS Catal. 2015, 5, 1804–1825. [Google Scholar] [CrossRef]
- Lu, J.; Elam, J.W.; Stair, P.C. Synthesis and stabilization of supported metal catalysts by atomic layer deposition. Acc. Chem. Res. 2013, 46, 1806–1815. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.T.; Feng, H.; Libera, J.L.; Guo, N.; Miller, J.T.; Stair, P.C.; Elam, J.W. Supported Ru− Pt bimetallic nanoparticle catalysts prepared by atomic layer deposition. Nano Lett. 2010, 10, 3047–3051. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.J.; Verheijen, M.A.; Bol, A.A.; Kessels, W.M.M. Sub-nanometer dimensions control of core/shell nanoparticles prepared by atomic layer deposition. Nanotechnology 2015, 26, 94002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weber, M.J.; Mackus, A.J.M.; Verheijen, M.A.; van der Marel, C.; Kessels, W.M.M. Supported Core/Shell Bimetallic Nanoparticles Synthesis by Atomic Layer Deposition. Chem. Mater. 2012, 24, 2973–2977. [Google Scholar] [CrossRef]
- Feng, H.; Elam, J.W.; Libera, J.A.; Setthapun, W.; Stair, P.C. Palladium catalysts synthesized by atomic layer deposition for methanol decomposition. Chem. Mater. 2010, 22, 3133–3142. [Google Scholar] [CrossRef]
- Lu, J.; Stair, P.C. Low-temperature ABC-type atomic layer deposition: Synthesis of highly uniform ultrafine supported metal nanoparticles. Angew. Chem. Int. Ed. 2010, 49, 2547–2551. [Google Scholar] [CrossRef] [PubMed]
- Assaud, L.; Brazeau, N.; Barr, M.K.S.; Hanbucken, M.; Ntais, S.; Baranova, E.A.; Santinacci, L. Atomic layer deposition of Pd nanoparticles on TiO2 nanotubes for ethanol electrooxidation: Synthesis and electrochemical properties. ACS Appl. Mater. Interfaces 2015, 7, 24533–24542. [Google Scholar] [CrossRef] [PubMed]
- Ten Eyck, G.A.; Pimanpang, S.; Bakhru, H.; Lu, T.; Wang, G. Atomic layer deposition of Pd on an oxidized metal substrate. Chem. Vap. Depos. 2006, 12, 290–294. [Google Scholar] [CrossRef]
- Barr, M.K.S.; Assaud, L.; Brazeau, N.; Hanbucken, M.; Ntais, S.; Santinacci, L.; Baranova, E.A. Enhancement of Pd catalytic activity toward ethanol electrooxidation by atomic layer deposition of SnO2 onto TiO2 Nanotubes. J. Phys. Chem. C 2017, 121, 17727–17736. [Google Scholar] [CrossRef]
- Assaud, L.; Monyoncho, E.; Pitzschel, K.; Allagui, A.; Petit, M.; Hanbücken, M.; Baranova, E.A.; Santinacci, L. 3D-nanoarchitectured Pd/Ni catalysts prepared by atomic layer deposition for the electrooxidation of formic acid. Beilstein J. Nanotechnol. 2014, 5, 162. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Qin, L.; Zhang, W.; Wan, H.; Lu, J.; Feng, H. Activated carbon supported palladium nanoparticle catalysts synthesized by atomic layer deposition: Genesis and evolution of nanoparticles and tuning the particle size. J. Phys. Chem. C 2015, 119, 11544–11556. [Google Scholar] [CrossRef]
- Rikkinen, E.; Santasalo-Aarnio, A.; Airaksinen, S.; Borghei, M.; Viitanen, V.; Sainio, J.; Kauppinen, E.I.; Kallio, T.; Krause, A.O.I. Atomic layer deposition preparation of Pd nanoparticles on a porous carbon support for alcohol oxidation. J. Phys. Chem. C 2011, 115, 23067–23073. [Google Scholar] [CrossRef]
- Liang, X.; Lyon, L.B.; Jiang, Y.-B.; Weimer, A.W. Scalable synthesis of palladium nanoparticle catalysts by atomic layer deposition. J. Nanoparticles Res. 2012, 14, 943. [Google Scholar] [CrossRef]
- Weber, M.; Collot, P.; El Gaddari, H.; Tingry, S.; Bechelany, M.; Holade, Y. Enhanced catalytic glycerol oxidation activity enabled by activated-carbon-supported palladium catalysts prepared through atomic layer deposition. Chem. Electro. Chem. 2018, 5, 743–747. [Google Scholar] [CrossRef]
- Grillo, F.; Moulijn, J.A.; Kreutzer, M.T.; van Ommen, J.R. Nanoparticle sintering in atomic layer deposition of supported catalysts: Kinetic modeling of the size distribution. Catal. Today 2018, 316, 51–61. [Google Scholar] [CrossRef]
- Grillo, F.; Van Bui, H.; Moulijn, J.A.; Kreutzer, M.T.; van Ommen, J.R. Understanding and controlling the aggregative growth of platinum nanoparticles in atomic layer deposition: An avenue to size selection. J. Phys. Chem. Lett. 2017, 8, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Langereis, E.; Heil, S.B.S.; Knoops, H.C.M.; Keuning, W.; Van de Sanden, M.C.M.; Kessels, W.M.M. In situ spectroscopic ellipsometry as a versatile tool for studying atomic layer deposition. J. Phys. D Appl. Phys. 2009, 42, 73001. [Google Scholar] [CrossRef]
- Weber, M.; Coy, E.; Iatsunskyi, I.; Yate, L.; Miele, P.; Bechelany, M. Mechanical properties of boron nitride thin films prepared by atomic layer deposition. CrystEngComm 2017, 19, 6089–6094. [Google Scholar] [CrossRef]
- Elam, J.W.; Zinovev, A.; Han, C.Y.; Wang, H.H.; Welp, U.; Hryn, J.N.; Pellin, M.J. Atomic layer deposition of palladium films on Al2O3 surfaces. Thin Solid Films 2006, 515, 1664–1673. [Google Scholar] [CrossRef]
- Goldstein, D.N.; George, S.M. Enhancing the nucleation of palladium atomic layer deposition on Al2O3 using trimethylaluminum to prevent surface poisoning by reaction products. Appl. Phys. Lett. 2009, 95, 143106. [Google Scholar] [CrossRef]
- Goldstein, D.N.; George, S.M. Surface poisoning in the nucleation and growth of palladium atomic layer deposition with Pd (hfac) 2 and formalin. Thin Solid Films 2011, 519, 5339–5347. [Google Scholar] [CrossRef]
- Postole, G.; Bonnetot, B.; Gervasini, A.; Guimon, C.; Auroux, A.; Ionescu, N.I.; Caldararu, M. Characterisation of BN-supported palladium oxide catalyst used for hydrocarbon oxidation. Appl. Catal. A Gen. 2007, 316, 250–258. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray photoelectron spectroscopy: A reference book of standard spectra for identification and interpretation of XPS data; Perkin-Elmer Corporation: Waltham, MA, USA, 1995. [Google Scholar]
- Shafeev, G.A.; Themlin, J.; Bellard, L.; Marine, W.; Cros, A. Enhanced adherence of area-selective electroless metal plating on insulators. J. Vac. Sci. Technol. 1996, 14, 319–326. [Google Scholar] [CrossRef]
- Grillo, F.; Van Bui, H.; La Zara, D.; Aarnink, A.A.I.; Kovalgin, A.Y.; Kooyman, P.; Kreutzer, M.T.; van Ommen, J.R. From Single Atoms to Nanoparticles: Autocatalysis and Metal Aggregation in Atomic Layer Deposition of Pt on TiO2 Nanopowder. Small 2018, 14, 1800765. [Google Scholar] [CrossRef] [PubMed]
- Jose-Yacaman, M.; Gutierrez-Wing, C.; Miki, M.; Yang, D.-Q.; Piyakis, K.N.; Sacher, E. Surface diffusion and coalescence of mobile metal nanoparticles. J. Phys. Chem. B 2005, 109, 9703–9711. [Google Scholar] [CrossRef] [PubMed]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, M.; Lamboux, C.; Navarra, B.; Miele, P.; Zanna, S.; Dufond, M.E.; Santinacci, L.; Bechelany, M. Boron Nitride as a Novel Support for Highly Stable Palladium Nanocatalysts by Atomic Layer Deposition. Nanomaterials 2018, 8, 849. https://doi.org/10.3390/nano8100849
Weber M, Lamboux C, Navarra B, Miele P, Zanna S, Dufond ME, Santinacci L, Bechelany M. Boron Nitride as a Novel Support for Highly Stable Palladium Nanocatalysts by Atomic Layer Deposition. Nanomaterials. 2018; 8(10):849. https://doi.org/10.3390/nano8100849
Chicago/Turabian StyleWeber, Matthieu, Cassandre Lamboux, Bruno Navarra, Philippe Miele, Sandrine Zanna, Maxime E. Dufond, Lionel Santinacci, and Mikhael Bechelany. 2018. "Boron Nitride as a Novel Support for Highly Stable Palladium Nanocatalysts by Atomic Layer Deposition" Nanomaterials 8, no. 10: 849. https://doi.org/10.3390/nano8100849
APA StyleWeber, M., Lamboux, C., Navarra, B., Miele, P., Zanna, S., Dufond, M. E., Santinacci, L., & Bechelany, M. (2018). Boron Nitride as a Novel Support for Highly Stable Palladium Nanocatalysts by Atomic Layer Deposition. Nanomaterials, 8(10), 849. https://doi.org/10.3390/nano8100849