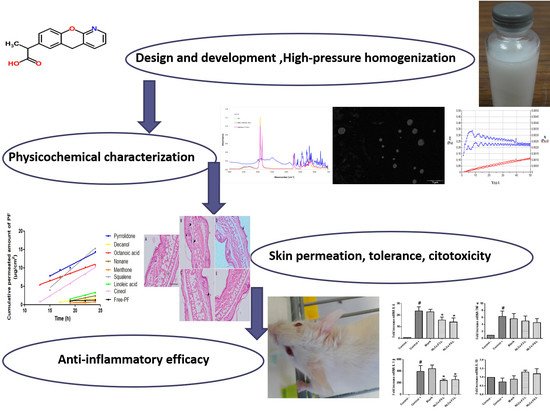
Development of Pranoprofen Loaded Nanostructured Lipid Carriers to Improve Its Release and Therapeutic Efficacy in Skin Inflammatory Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of NLCs
2.2.2. Design of Experiments
2.2.3. Physicochemical Characterization
Particle Size and Zeta Potential
Entrapment Efficiency
Transmission Electron Microscopy
X-ray Spectroscopy (XRD)
Fourier transform infrared (FTIR)
Extensibility (Spreadability)
Rheology
2.2.4. Stability
2.2.5. In Vitro Studies
Release
Cell Viability Assay
2.2.6. Ex Vivo Studies
Human Skin Permeation Assay
Histological Analysis
2.2.7. In Vivo Assays
Skin Integrity Parameters
Anti-Inflammatory Efficacy
Transcriptional Analysis of Key Inflammatory Biomarkers
Skin Irritation Testing
3. Results
3.1. Design of Experiments and Physicochemical Characterization
3.1.1. Transmission Electron Microscopy (TEM)
3.1.2. X-ray Spectroscopy (XRD)
3.1.3. Fourier Transform Infrared (FTIR)
3.1.4. Extensibility (Spreadability)
3.1.5. Rheological Studies
3.2. Stability Studies
3.3. In Vitro Release Study
3.4. In Vitro Cell Viability Assay
3.5. Human Skin Permeation Studies
3.6. Histological Analysis
3.7. Model of Mice Ear Inflammation Induced with TPA
3.8. In Vivo Mice Model and Inflammatory Response after the NLCs-F3-L and NLCs-F9-L Treatment
3.9. Skin Integrity Parameters
3.10. Skin Irritation Testing
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vaughn, A.R.C.; Clark, A.K.; Sivamani, R.K.; Shi, V.Y. Natural Oils for Skin-Barrier Repair: Ancient Compounds Now Backed by Modern Science. Am. J. Cin. Dermatol. 2018, 19, 103–117. [Google Scholar] [CrossRef] [PubMed]
- Moner, V.; Fernandez, E.; Calpena, A.C.; Garcia-Herrera, A.; Cocera, M.; Lopez, O. A lamellar body mimetic system for the treatment of oxazolone-induced atopic dermatitis in hairless mice. J. Dermatol. Sci. 2018, 90, 172–179. [Google Scholar] [CrossRef] [PubMed]
- RodriguesLeite-Silva, V.; Mandelli de Almeida, M.; Fradin, A.; Grice, J.E.; Stephen, M. Delivery of drugs applied topically to the skin. Expert Rev. Dermatol. 2012, 7, 383–397. [Google Scholar] [CrossRef] [Green Version]
- Asbill, C.S.; Michniak, B.B. Percutaneus penetration enhancers: Local versus trasdermal activity. Pharm. Sci. Technol. Today 2000, 3, 36–41. [Google Scholar] [CrossRef]
- Abdel-Mottaleb, M.M.; Try, C.; Pellequer, Y.; Lamprecht, A. Nanomedicine strategies for targeting skin inflammation. Nanomedicine. 2014, 9, 1727–1743. [Google Scholar] [CrossRef]
- Vogt, A.; Wischke, C.; Neffe, A.T.; Ma, N.; Alexiev, U.; Lendlein, A. Nanocarriers for drug delivery into and through the skin—Do existing technologies match clinical challenges? J. Control. Release 2016, 242, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Cañadas-Enrich, C.; Abrego, G.; Alvarado, H.L.; Calpena, A.C.; Boix-Montañes, A. Pranoprofen quantification in ex vivo corneal and scleral permeation samples: Analytical validation. J. Pharm. Biomed. Anal. 2018, 160, 109–118. [Google Scholar] [CrossRef]
- Abrego, G.; Alvarado, H.; Souto, E.B.; Guevara, B.; Bellowa, L.H.; Garduno, M.L.; Garcia, M.L.; Calpena, A.C. Biopharmaceutical profile of hydrogels containing pranoprofen-loaded PLGA nanoparticles for skin administration: In vitro, ex vivo and in vivo characterization. Int. J. Pharm. 2016, 501, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Abrego, G.; Alvarado, H.; Souto, E.B.; Guevara, B.; Bellowa, L.H.; Parra, A.; Calpena, A.; Garcia, M.L. Biopharmaceutical profile of pranoprofen-loaded PLGA nanoparticles containing hydrogels for ocular administration. Eur. J. Pharm. Biopharm. 2015, 95, 261–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobe, K.; Otsu, H.; Kobe, Y.; Kakogawa, Y. Method for Stabilizing Pranoprofen and Stable Liquid Preparation of Pranoprofen. U.S. Patent US005856345A, 1999. [Google Scholar]
- Abrego, G.; Alvarado, H.L.; Egea, M.A.; Gonzalez-Mira, E.; Calpena, A.C.; Garcia, M.L. Design of nanosuspensions and freeze-dried PLGA nanoparticles as a novel approach for ophthalmic delivery of pranoprofen. J. Pharm. Sci. 2014, 103, 3153–3164. [Google Scholar] [CrossRef]
- Carvajal-Vidal, P.; Mallandrich, M.; Garcia, M.L.; Calpena, A.C. Effect of Different Skin Penetration Promoters in Halobetasol Propionate Permeation and Retention in Human Skin. Int. J. Mol. Sci. 2017, 18, 2475. [Google Scholar] [CrossRef]
- Morales, J.O.; Valdés, K.; Morales, J.; Oyarzun-Ampuero, F. Lipid nanoparticles for the topical delivery of retinoids and derivatives. Nanomedicine. 2015, 10, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Fangeiro, J.F.; Andreani, T.; Egea, M.A.; Garcia, M.L.; Souto, S.B.; Souto, E.B. Experimental factorial design applied to mucoadhesive lipid nanoparticles via multiple emulsion process. Colloids Surf. B Biointerfaces 2012, 100, 84–89. [Google Scholar] [CrossRef]
- Araújo, J.; Garcia, M.L.; Mallandrich, M.; Souto, E.B.; Calpena, A.C. Release profile and transscleral permeation of triamcinolone acetonide loaded nanostructured lipid carriers (TA-NLC): In vitro and ex vivo studies. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Ettcheto, M.; Egea, M.A.; Espina, M.; Calpena, A.C.; Folch, J.; Camins, A.; García, M.L. New potential strategies for Alzheimer‘s disease prevention: Pegylated biodegradable dexibuprofen nanospheres administration to APPswe/PS1dE9. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mira, E.; Egea, M.A.; Garcia, M.L.; Souto, E.B. Design and ocular tolerance of flurbiprofen loaded ultrasound-engineered NLC. Colloids Surf. B Biointerfaces 2010, 81, 412–421. [Google Scholar] [CrossRef]
- Araújo, J.; Vega, E.; Lopes, C.; Egea, M.A.; Garcia, M.L.; Souto, E.B. Effect of polymer viscosity on physicochemical properties and ocular tolerance of FB-loaded PLGA nanospheres. Colloids Surf. B Biointerfaces 2009, 72, 48–56. [Google Scholar] [CrossRef]
- Silva-Abreu, M.; Calpena, A.C.; Andrés-Benito, P.; Aso, E.; Romero, I.A.; Roig-Carles, D. PPAR agonist-loaded PLGA-PEG nanocarriers as a potential treatment for Alzheimer’s disease: In vitro and in vivo studies. Int. J. Nanomed. 2018, 13, 5577–5590. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mira, E.; Egea, M.A.; Souto, E.B.; Calpena, A.C.; Garcia, M.L. Optimizing flurbiprofen-loaded NLC by central composite factorial design for ocular delivery. Nanotechnology 2011, 22, 045101. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, H.L.; Abrego, G.; Souto, E.B.; Garduno-Ramirez, M.L.; Clares, B.; Garcia, M.L.; Calpena, A.C. Nanoemulsions for dermal controlled release of oleanolic and ursolic acids: In vitro, ex vivo and in vivo characterization. Colloids Surf. B Biointerfaces 2015, 130, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Egea, M.A.; Cano, A.; Espina, M.; Calpena, A.C.; Ettcheto, M.; Camins, A.; Souto, E.B.; Silva, A.M.; García, M.L. PEGylated PLGA nanospheres optimized by design of experiments for ocular administration of dexibuprofen—in vitro, ex vivo and in vivo characterization. Colloids Surf. B Biointerfaces 2016, 145, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Suñer, J.; Calpena, A.C.; Clares, B.; Cañadas, C.; Halbaut, L. Development of Clotrimazole Multiple W/O/W Emulsions as Vehicles for Drug Delivery: Effects of Additives on Emulsion Stability. AAPS PharmSciTech 2017, 18, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; González-Mira, E.; García, M.L.; Egea, M.A.; Fonseca, J.; Silva, R.C.; Santos, D.; Souto, E.B.; Ferreira, D. Preparation, characterization and biocompatibility studies on risperidona-loaded solid lipid nanoparticles (SLN): High pressure homogenization versus ultrasound. Colloids Surf. B Biointerfaces 2011, 86, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Costa-Lima, S.; Carneiro, T.; Cordeiro-da-Silva, A.; Souto, E.B.; Ferreira, D.C. Solid lipid nanoparticles as intracellular drug transporters: An investigation of the uptake mechanism and pathway. Int. J. Pharm. 2012, 430, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Ramos Yacasi, G.R.; García, M.L.; Espina, M.; Parra, A.; Calpena, A.C. Influence of freeze-drying and γ-irradation in preclinicalstudies of flurbiprofen polymeric nanoparticles for ocular delivery using D-(+)-trehalose and polyethylene glycol. Int. J. Nanomed. 2016, 11, 4093–4106. [Google Scholar] [CrossRef] [PubMed]
- Mulik, R.S.; Mönkkönen, J.; Juvonen, R.O.; Mahadik, K.R.; Paradkar, A.R. Transferrin mediated solid lipid nanoparticles containing curcumin: Enhanced in vitro anticancer activity by induction of apoptosis. Int. J. Pharm. 2010, 398, 190–203. [Google Scholar] [CrossRef]
- Mallandrich, M.; Fernández Campos, F.; Clares, B.; Halbaut, L.; Alonso, C.; Coderch, L.; Garduño-Ramírez, M.L.; Andrade, B.; del Pozo, A.; Lane, M.E. Developing transdermal applications of ketorolac tromethamine entrapped in stimuli sensitive block copolymer hydrogels. Pharm. Res. 2017, 34, 1728–1740. [Google Scholar] [CrossRef]
- World Medical Association. World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Dominguez-Villegas, V.; Clares Naveros, B.; García Lopez, M.L.; Calpena-Campany, A.C.; Bustos-Zagal, P.; Garduño-Ramirez, M.L. Development and charazterization of two nano-structured systems for topical application of flavanones isolated from Eysenhardtia Platycarpa. Colloids Surf. B Biointerfaces 2014, 116, 183–192. [Google Scholar] [CrossRef]
- Parra, A.; Clares, B.; Rossello, A.; Garduno-Ramirez, M.L.; Abrego, G.; Garcia, M.L.; Calpena, A.C. Ex vivo permeation of carprofen from nanoparticles: A comprehensive study through human, porcine and bovine skin as anti-inflammatory agent. Int. J. Pharm. 2016, 501, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Draize, J.; Woodard, G.; Calvery, H. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. 1994, 82, 377–390. [Google Scholar]
- Parra, A.; Mallandrich, M.; Clares, B.; Egea, M.A.; Espina, M.; Garcia, M.L.; Calpena, A.C. Design and elaboration of freeze-dried PLGA nanoparticles for the transcorneal permeation of carprofen: Ocular anti-inflammatory applications. Colloids Surf. B Biointerfaces 2015, 136, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Doherty, N.S.; Beaver, T.H.; Rheins, L.A.; Nordlund, J.J. Multiple topical applications of arachidonic acid to mouse ears induce inflammatory and proliferative changes. J. Investig. Dermatol. 1988, 91, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Grewe, M.; Gyufko, K.; Krutmann, J. Interleukin-10 production by cultured human keratinocytes: Regulation by ultraviolet B and ultraviolet A1 radiation. J. Investig. Dermatol. 1995, 104, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mira, E.; Nikolic, S.; Garcia, M.L.; Egea, M.A.; Souto, E.B.; Calpena, A.C. Potential use of nanostructured lipid carriers for topical delivery of flurbiprofen. J. Pharm. Sci. 2011, 100, 242–251. [Google Scholar] [CrossRef]
- Rincón, M.; Calpena, A.C.; Clares, B.; Espina, M.; Garduño-Ramírez, M.L.; Rodríguez-Lagunas, M.J.; García, M.L.; Abrego, G. Skin-controlled release lipid nanosystems of pranoprofen for the treatment of localinflammation and pain. Nanomedicine. 2018, 13, 2397–2413. [Google Scholar] [CrossRef] [PubMed]
- Martín-Villena, M.; Fernández Campos, F.; Calpena-Campany, A.; Bozal-de FeFebrer, N.; Ruíz Martinez, M.; Clares-Naveros, B. Novel microparticulate systems for the vaginal delivery of nystatin: Development and characterization. Carbohydr. Polym. 2013, 94, 1–11. [Google Scholar] [CrossRef]
- Fang, J.; Fang, C.; Liu, C.; Su, Y. Lipid nanoparticles as vehicles for topical psoralen delivery: Solid lipid nanoparticles (SLN) versus nanostructured lipid carriers (NLC). Eur. J. Pharm. Biopharm. 2008, 70, 633–640. [Google Scholar] [CrossRef]
- Silva-Abreu, M.; Espinoza, L.C.; Rodriguez-Lagunas, M.J.; Fabrega, M.J.; Espina, M.; Garcia, M.L.; Calpena, A.C. Human Skin Permeation Studies with PPARgamma Agonist to Improve Its Permeability and Efficacy in Inflammatory Processes. Int. J. Mol. Sci. 2017, 18, 2548. [Google Scholar] [CrossRef]
- Alvarado, H.L.; Abrego, G.; Garduño-Ramirez, M.L.; Clares, B.; Calpena, A.C.; García, M.L. Design and optimization of oleanolic/ursolic acid-loaded nanoplatforms for ocular anti-inflammatory applications. Nanomed. Nanotechnol. Biol. Med.. 2015, 1, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.C.; Lee, C.W.; Tsai, M.H.; Ko, H.H.; Fang, J.Y.; Chiang, Y.C.; Liang, C.J.; Hsu, L.F.; Hu, S.C.; Yen, F.L. Eupafolin nanoparticles protect HaCaT keratinocytes from particulate matter-infuced inflammation and oxidative stress. Int. J. Nanomed. 2016, 11, 3907–3926. [Google Scholar]
- Crosera, M.; Prodi, A.; Mauro, M.; Pelin, M.; Florio, C.; Bellomo, F.; Adami, G.; Apostoli, P.; De Palma, G.; Bovenzi, M.; et al. Titanium dioxide nanoparticle permetration into the skin and effects of HaCaT cells. Int. J. Environ. Res. Public Health 2015, 12, 9282–9297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graham, S.; Phillip, R.; Zahid, M.; Bano, N.; Iqbal, Q.; Mahboob, F.; Chen, X.; Shang, L. Ibuprofen nanoparticles and its cytotoxicity on A549 and HaCaT cell lines. In Proceedings of the Physiological Society: Respiratory Abstracts. 37, PCA138; Physiological Society: Dublin, Ireland, 2016. [Google Scholar]
- Wissing, S.A.; Kayser, O.; Muller, R.H. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Mussi, S.V.; Silva, R.C.; Oliveira, M.C.; Lucci, C.M.; Azevedo, R.B.; Ferreira, L.A. New approach to improve encapsulation and antitumor activity of doxorubicin loaded in solid lipid nanoparticles. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2013, 48, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Sosa, L.; Clares, B.; Alvarado, H.L.; Bozal, N.; Domenech, O.; Calpena, A.C. Amphotericin B releasing topical nanoemulsion for the treatment of candidiasis and aspergillosis. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2303–2312. [Google Scholar] [CrossRef] [PubMed]
- Schäfer-Korting, M.; Mehnert, W.; Korting, H.C. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv. Drug Deliv. Rev. 2007, 59, 427–443. [Google Scholar] [CrossRef]
- Doktorovova, S.; Kovačević, A.B.; Garcia, M.L.; Souto, E.B. Preclinical safety of solid lipid nanoparticles and nanostuctured lipid carriers: Current evidence from in vitro and in vivo evaluation. Eur. J. Pharm. Biopharm. 2016, 108, 235–252. [Google Scholar] [CrossRef]














| Composition | Physicochemical Characterization | |||||||
|---|---|---|---|---|---|---|---|---|
| cPF (%) | cSL/L (%) | cTW (%) | Linoleic Acid (%) | Z-Ave (nm) ± SD | PI ± SD | ZP (mV) ± SD | EE (%) ± SD | |
| NLCs-F3 | 1.50 | 50.00 | 2.50 | - | 248.40 ± 6.34 | 0.229 ± 0.046 | −10.70 ± 0.44 | 99.68 ± 0.17 |
| NLCs-F3-L | 1.50 | 50.00 | 2.50 | 5 | 274.59 ± 8.37 | 0.255 ± 0.077 | −9.89 ± 0.50 | 97.96 ± 0.24 |
| NLCs-F9 | 1.00 | 76.80 | 3.00 | - | 222.30 ± 9.74 | 0.237 ± 0.085 | −9.20 ± 0.48 | 99.61 ± 0.12 |
| NLCs-F9-L | 1.00 | 76.80 | 3.00 | 5 | 266.98 ± 9.82 | 0.280 ± 0.039 | −10.01 ± 0.36 | 98.37 ± 0.28 |
| A NLCs-F3 | |||||
| Time (days) | Temperature | Z-Ave (nm) ± SD | PI± SD | ZP (mV) ± SD | EE (%) |
| 1 | 4 °C | 249.00 ± 4.95 | 0.29 ± 0.05 | −10.63 ± 0.08 | 99.62 |
| 3 | 249.60 ± 8.85 | 0.30 ± 0.04 | −10.75 ± 0.48 | 99.59 | |
| 8 | 265.77 ± 8.77 | 0.29 ± 0.02 | −10.46 ± 0.17 | 99.08 | |
| 15 | 269.30 ± 3.23 | 0.24 ± 0.02 | −9.84 ± 0.16 | 98.90 | |
| 30 | 278.80 ± 5.87 | 0.33 ± 0.04 | −8.99 ± 0.64 | 98.86 | |
| 60 | 290.47 ± 3.33 | 0.35 ± 0.06 | −9.05 ± 0.54 | 98.79 | |
| 90 | 306.52 ± 6.02 | 0.27 ± 0.08 | −9.00 ± 0.26 | 98.23 | |
| 180 | 314.99 ± 5.77 | 0.30 ± 0.08 | −8.98 ± 0.34 | 98.00 | |
| 1 | 25 °C | 248.65 ± 5.12 | 0.23 ± 0.04 | −10.62 ± 0.29 | 99.68 |
| 3 | 249.60 ± 6.40 | 0.23 ± 0.03 | −10.53 ± 0.34 | 99.07 | |
| 8 | 279.87 ± 5.59 | 0.25 ± 0.02 | −10.60 ± 0.33 | 98.33 | |
| 15 | 308.43 ± 4.15 | 0.23 ± 0.05 | −10.68 ± 0.40 | 98.68 | |
| 30 | 325.20 ± 6.86 | 0.26 ± 0.02 | −10.16 ± 0.12 | 98.84 | |
| 60 | 378.30 ± 3.68 | 0.20 ± 0.03 | −9.80 ± 0.15 | 98.06 | |
| 90 | 410.38 ± 4.01 | 0.33 ± 0.06 | −9.33 ± 0.26 | 98.02 | |
| 180 | 478.40 ± 6.25 | 0.28 ± 0.03 | −9.35 ± 0.19 | 97.38 | |
| 1 | 37 °C | 253.77 ± 4.66 | 0.27 ± 0.03 | −10.59 ± 0.41 | 99.67 |
| 3 | 499.54 ± 6.34 | 0.28 ± 0.02 | −10.60 ± 0.22 | 99.66 | |
| 8 | 700.45 ± 6.67 | 0.27 ± 0.05 | −10.30 ± 0.28 | 98.01 | |
| 15 | 1134.22 ± 29.62 | 0.39 ± 0.09 | −9.99 ± 0.36 | 98.01 | |
| 30 | 1998.05 ± 26.89 | 0.45 ± 0.08 | −10.50 ± 0.35 | 97.60 | |
| 60 | 2467.20 ± 12.11 | 0.45 ± 0.05 | −10.42 ± 0.63 | 97.43 | |
| 90 | 3001.06 ± 49.94 | 0.47 ± 0.03 | −10.51 ± 0.50 | 97.33 | |
| 180 | - | - | −10.56 ± 0.21 | 96.98 | |
| B NLCs-F3-L | |||||
| Time (days) | Temperature | Z-Ave (nm) ± SD | PI ± SD | ZP (mV) ± SD | EE (%) |
| 1 | 4 °C | 225.30± 8.02 | 0.29 ± 0.03 | −9.34 ± 0.11 | 99.03 |
| 3 | 256.44± 6.95 | 0.29 ± 0.04 | −9.35 ± 0.48 | 98.98 | |
| 8 | 268.90 ± 9.75 | 0.31 ± 0.03 | −9.16 ± 0.07 | 99.03 | |
| 15 | 273.13 ± 5.10 | 0.33 ± 0.06 | −8.48 ± 0.20 | 98.85 | |
| 30 | 276.60 ± 7.92 | 0.34 ± 0.05 | −8.81 ± 0.76 | 98.84 | |
| 60 | 280.30 ± 3.15 | 0.29 ± 0.06 | −8.21 ± 0.53 | 98.86 | |
| 90 | 296.72 ± 6.02 | 0.28 ± 0.07 | −8.40 ± 0.36 | 98.12 | |
| 180 | 299.98 ± 4.68 | 0.33 ± 0.06 | −8.33 ± 0.31 | 97.35 | |
| 1 | 25 °C | 222.90 ± 4.94 | 0.24 ± 0.01 | −9.80 ± 0.28 | 98.93 |
| 3 | 256.54 ± 4.70 | 0.25 ± 0.03 | −9.39 ± 0.25 | 98.84 | |
| 8 | 280.82 ± 2.40 | 0.24 ± 0.02 | −9.86 ± 0.23 | 98.80 | |
| 15 | 307.00 ± 4.15 | 0.24 ± 0.03 | −9.68 ± 0.34 | 98.87 | |
| 30 | 328.10 ± 6.86 | 0.28 ± 0.04 | −9.16 ± 0.12 | 98.73 | |
| 60 | 357.20 ± 2.89 | 0.29 ± 0.02 | −9.12 ± 0.15 | 98.91 | |
| 90 | 400.04 ± 5.01 | 0.27 ± 0.07 | −8.19 ± 0.22 | 98.80 | |
| 180 | 423.56 ± 8.01 | 0.28 ± 0.05 | −8.51 ± 0.16 | 97.08 | |
| 1 | 37 °C | 223.50 ± 3.97 | 0.29 ± 0.03 | −9.90 ± 0.43 | 98.99 |
| 3 | 497.70 ± 6.76 | 0.31 ± 0.02 | −9.10 ± 0.23 | 99.07 | |
| 8 | 703.11 ± 7.17 | 0.29 ± 0.05 | −10.30 ± 0.36 | 98.95 | |
| 15 | 1019.00 ± 22.32 | 0.30 ± 0.03 | −9.45 ± 0.32 | 98.87 | |
| 30 | 1654.86 ± 15.21 | 0.38 ± 0.04 | −9.48 ± 0.14 | 99.02 | |
| 60 | 2007.67 ± 9.94 | 0.37 ± 0.06 | −10.00 ± 0.14 | 98.57 | |
| 90 | 2700.01 ± 44.12 | 0.39 ± 0.07 | −10.10 ± 0.25 | 98.95 | |
| 180 | 5002.07 ± 234.45 | 0.48 ± 0.06 | −9.30 ± 0.43 | 97.02 | |
| C NLCs-F-9 | |||||
| Time (days) | Temperature | Z-Ave (nm) ± SD | PI ± SD | ZP (mV) ± SD | EE (%) |
| 1 | 4 °C | 242.07 ± 3.93 | 0.24 ± 0.03 | −10.48 ± 0.27 | 99.06 |
| 3 | 242.89 ± 6.30 | 0.26 ± 0.02 | −10.85 ± 0.50 | 98.82 | |
| 8 | 260.90 ± 9.75 | 0.30 ± 0.05 | −9.86 ± 0.16 | 99.12 | |
| 15 | 276.10 ± 2.72 | 0.26 ± 0.03 | −9.48 ± 0.09 | 98.81 | |
| 30 | 257.02 ± 5.42 | 0.27 ± 0.02 | −8.71 ± 0.22 | 98.80 | |
| 60 | 263.30 ± 1.25 | 0.29 ± 0.02 | −9.21 ± 0.19 | 98.78 | |
| 90 | 300.74 ± 5.69 | 0.29 ± 0.06 | −9.00 ± 0.23 | 98.50 | |
| 180 | 320.22 ± 7.43 | 0.33 ± 0.05 | −8.12 ± 0.29 | 97.35 | |
| 1 | 25 °C | 240.75 ± 3.69 | 0.25 ± 0.03 | −10.70 ± 0.23 | 98.99 |
| 3 | 249.55 ± 6.21 | 0.25 ± 0.01 | −10.61 ± 0.24 | 98.82 | |
| 8 | 286.52 ± 4.02 | 0.27 ± 0.02 | −10.62 ± 0.30 | 98.79 | |
| 15 | 299.06 ± 3.84 | 0.26 ± 0.02 | −10.54 ± 0.34 | 98.88 | |
| 30 | 310.09 ± 5.18 | 0.27 ± 0.01 | −9.76 ± 0.22 | 98.84 | |
| 60 | 339.56 ± 5.16 | 0.27 ± 0.01 | −9.68 ± 0.20 | 98.81 | |
| 90 | 411.47 ± 4.02 | 0.28 ± 0.08 | −9.50 ± 0.18 | 98.81 | |
| 180 | 459.60 ± 6.20 | 0.29 ± 0.05 | −9.32 ± 0.14 | 97.08 | |
| 1 | 37 °C | 244.99 ± 2.55 | 0.24 ± 0.02 | −10.60 ± 0.47 | 99.00 |
| 3 | 487.54 ± 8.04 | 0.25 ± 0.01 | −10.68 ± 0.22 | 99.06 | |
| 8 | 698.99 ± 8.89 | 0.29 ± 0.06 | −10.55 ± 0.33 | 98.84 | |
| 15 | 1076.04 ± 5.52 | 0.30 ± 0.02 | −9.97 ± 0.36 | 98.89 | |
| 30 | 1673.13 ± 17.08 | 0.33 ± 0.04 | −10.18 ± 0.21 | 99.02 | |
| 60 | 2197.46 ± 9.89 | 0.34 ± 0.02 | −10.11 ± 0.26 | 98.63 | |
| 90 | 2998.34 ± 44.19 | 0.39 ± 0.05 | −10.30 ± 0.32 | 98.74 | |
| 180 | - | - | −11.11 ± 0.57 | 96.08 | |
| D NLCs-F9-L | |||||
| Time (days) | Temperature | Z-Ave (nm) ± SD | PI ± SD | ZP (mV) ± SD | EE (%) |
| 1 | 4 °C | 232.62 ± 3.20 | 0.28 ± 0.02 | −9.74 ± 0.23 | 98.97 |
| 3 | 239.90 ± 1.97 | 0.30 ± 0.03 | −9.85 ± 0.44 | 98.61 | |
| 8 | 242.76 ± 5.23 | 0.27 ± 0.04 | −9.06 ± 0.19 | 98.94 | |
| 15 | 246.10 ± 2.72 | 0.28 ± 0.01 | −8.98 ± 0.20 | 98.80 | |
| 30 | 253.80 ± 5.49 | 0.31 ± 0.02 | −8.71 ± 0.22 | 98.89 | |
| 60 | 259.57 ± 3.09 | 0.29 ± 0.05 | −8.61 ± 0.29 | 98.60 | |
| 90 | 280.83 ± 5.11 | 0.32 ± 0.03 | −8.20 ± 0.25 | 98.67 | |
| 180 | 301.85 ± 3.86 | 0.35 ± 0.07 | −7.98 ± 0.37 | 98.10 | |
| 1 | 25 °C | 231.54 ± 2.22 | 0.25 ± 0.02 | −10.00 ± 0.37 | 98.95 |
| 3 | 246.81 ± 3.18 | 0.24 ± 0.03 | −9.98 ± 0.25 | 98.75 | |
| 8 | 260.51 ± 2.66 | 0.26 ± 0.02 | −9.96 ± 0.22 | 98.75 | |
| 15 | 307.00 ± 4.15 | 0.27 ± 0.01 | −9.88 ± 0.29 | 99.08 | |
| 30 | 328.10 ± 6.86 | 0.29 ± 0.02 | −9.05 ± 0.13 | 98.89 | |
| 60 | 333.20 ± 3.68 | 0.30 ± 0.01 | −9.12 ± 0.16 | 98.78 | |
| 90 | 410.64 ± 2.97 | 0.29 ± 0.06 | −9.00 ± 0.18 | 98.50 | |
| 180 | 497.70 ± 5.75 | 0.32 ± 0.03 | −8.80 ± 0.16 | 97.39 | |
| 1 | 37 °C | 243.60 ± 4.96 | 0.27 ± 0.02 | −9.80 ± 0.39 | 98.99 |
| 3 | 300.70 ±.3.01 | 0.30 ± 0.02 | −9.80 ± 0.23 | 99.02 | |
| 8 | 676.23 ± 5.88 | 0.27 ± 0.02 | −9.92 ± 0.36 | 98.89 | |
| 15 | 1186.12 ± 21.03 | 0.28 ± 0.02 | −9.70 ± 0.26 | 98.88 | |
| 30 | 1967.03 ± 22.72 | 0.29 ± 0.02 | −9.86 ± 0.15 | 99.00 | |
| 60 | 2401.35 ± 23.00 | 0.30 ± 0.04 | −10.01 ± 0.34 | 98.58 | |
| 90 | 2884.10 ± 74.09 | 0.39 ± 0.03 | −9.76 ± 0.25 | 97.94 | |
| 180 | - | 0.41 ± 0.04 | −9.80 ± 0.52 | 94.32 | |
| Order Equation | Parameters | Unit | Value (Mean ± SD) | ||||
|---|---|---|---|---|---|---|---|
| NLCs-F3 | NLCs-F3-L | NLCs-F9 | NLCs-F9-L | ||||
| Zero order | Qt = K0 t + Q∞ | K0 | µg/h | 4.09 ± 0.34 | 4.21 ± 0.33 | 4.30 ± 0.56 | 4.10 ± 0.54 |
| Q∞ | µg | 17.48 ± 8.21 | 19.76 ± 8.26 | 35.11 ± 13.94 | 30.19 ± 13.26 | ||
| r2 | - | 0.9539 | 0.9559 | 0.8881 | 0.8887 | ||
| AIC | - | 71.53 | 71.61 | 79.92 | 79.11 | ||
| First order | Qt = Q∞(1-e−kf·t) | Kf | h−1 | 0.032 ± 0.009 | 0.034 ± 0.009 | 0.057 ± 0.015 | 0.053 ± 0.015 |
| Q∞ | µg | 239.20 ± 38.62 | 244.00 ± 36.70 | 217.7 ± 22.89 | 209.30 ± 25.63 | ||
| r2 | - | 0.9752 | 0.9757 | 0.9472 | 0.9438 | ||
| t ½ | h | 21.16 | 20.38 | 12.15 | 13.07 | ||
| AIC | - | 66.55 | 66.87 | 73.92 | 73.65 | ||
| Korsmeyer-Peppas | Qt = Kk t n | KK | h−n | 13.17± 3.27 | 14.42 ± 3.31 | 23.63 ± 6.96 | 20.47 ± 6.56 |
| n | - | 0.70 ± 0.07 | 0.69 ± 0.07 | 0.58 ± 0.09 | 0.60 ± 0.09 | ||
| r2 | - | 0.9727 | 0.9752 | 0.9323 | 0.9284 | ||
| AIC | - | 67.32 | 67.02 | 75.89 | 75.59 | ||
| Weibull | Qt = Q∞(1-e−(t/td)β) | td | h | 39.80 ± 25.51 | 52.50 ± 47.64 | 17.28 ± 4.94 | 17.55 ± 4.86 |
| β | - | 0.924 ± 0.238 | 0.859 ± 0.232 | 1.003 ± 0.287 | 1.048 ± 0.307 | ||
| Q∞ | µg | 276.2 ± 97.5 | 333.3 ± 165.7 | 217.2 ± 26.7 | 202.7 ± 24.9 | ||
| r2 | - | 0.9756 | 0.9769 | 0,9472 | 0.9440 | ||
| AIC | - | 68.43 | 68.43 | 75.92 | 75.62 | ||
| Penetration Enhancers | J (µg/(h/cm2)) | Kp (cm/h) × 105 | Qret (µg/g/cm2) | Q24h (µg) | Young Subject Css (µg/mL) × 103 | Elderly Subject Css (µg/mL) × 103 |
|---|---|---|---|---|---|---|
| Pyrrolidone | 0.780 i (0.600–0.800) | 111.0 i (94.0–125.0) | 94.61 i (89.00–99.00) | 13.00 i (12.10–14.32) | 96.41 (81.75–109.00) | 181.54 (153.94–205.25) |
| Decanol | 0.121 a,c,d,e,f,g,h,i (0.117–0.120) | 18.8 a,c,d,e,f,g,h,i (18.3–18.9) | 107.41 a,c,d,e,f,g,h,i (103.00–113.01) | 11.18 a,c,d,e,f,g,i (10.00–12.00) | 16.35 (15.99–16.47) | 30.78 (30.12–31.02) |
| Octanoic acid | 0.534 a,d,e,f,g,h,i (0.400–0.600) | 83.5 a,d,e,f,g,h (62.5–93.8) | 130.78 a,d,e,f,g,h,i (124.01–135) | 87.64 a,d,e,f,g,h,I (86.30–88.80) | 72.82 (54.50–81.75) | 137.13 (102.65–153.93) |
| Nonane | 0.841 e,f,g,i (0.700–0.950) | 130.0 e,f,g,i (110.0–150.0) | 65.22 a,f,g,h,i (62.00–68.02) | 13.02 e,f,g,h,i (12.40–14.50) | 114.59 (95.37–129.44) | 215.77 (179.59–243.73) |
| Menthone | 0.051 a,f,g,h (0.400–0.600) | 7.8 a,f,g,h,i (6.3–9.4) | 69.21 a,f,g,h,i (64.00–73.00) | 3.91 a,g,h,i (2.70–4.60) | 6.84 (5.45–8.17) | 12.88 (10.26–15.39) |
| Squalene | 1.203 a,g,h,i (1.100–1.300) | 190.0 a,g,h,i (170.0–200.0) | 40.81 a,g,h,i (37.00–45.02) | 15.22 a,g,h,i (13.90–16.30) | 163.91 (149.87–177.12) | 308.64 (282.22–333.53) |
| Linoleic acid | 0.362 a,i (0.200–0.400) | 56.6 a,i (31.3–62.5) | 194.99 a,h,i (190.00–197.00) | 3.31 a,h,i (2.10–4.60) | 49.34 (27.25–54.50) | 92.90 (51.31–102.62) |
| Cineol | 0.860 i (0.700–0.900) | 130.0 i (110.0–140.0) | 92.81 a,i (86.00–97.00) | 10.35 a,i (9.10–11.20) | 117.17 (95.37–122.62) | 220.64 (179.59–230.90) |
| Free-PF | 0.050 (0.040–0.060) | 79.0 (63.0–94.0) | 35.04 (31.30–36.21) | 1.23 (0.98–1.33) | 68.63 (54.50–81.75) | 129.23 (102.62–153.94) |
| Parameters | NLCs-F3 | NLCs-F3-L | NLCs-F9 | NLCs-F9-L |
|---|---|---|---|---|
| J × 102 mg/cm2h | 7.27 a (6.30–8.51) | 13.17 (11.14–14.27) | 7.45 b (6.38–8.48) | 9.11 (7.99–10.47) |
| Kp × 104 (cm/h)TL (h) QR (µg/cm2g) | 1.6 a (1.4–1.9) 1.67 a (1.23–1.99) 24.29 a (22.31–24.30) | 2.7 (2.3–3.0) 13.76 (12.74–14.78) 57.15 (57.14–57.16) | 2.5 (2.1–2.8) 13.41 b (13.35–13.43) 19.27 b (19.26–19.28) | 2.8 (2.5–3.3) 10.98 (8.95–11.99) 32.88 (32.86–32.89) |
| Formulation | Young Subject Css (µg/mL) × 103 | Elderly Subject Css (µg/mL) × 103 |
|---|---|---|
| NLCs-F3 | 8.60 (7.30–11.80) | 16.36 (13.75–22.25) |
| NLCs-F3-L | 1.06 (0.99–1.14) | 1.99 (1.85–2.15) |
| NLCs-F9 | 1.08 (1.07–1.09) | 2.04 (2.03–2.05) |
| NLCs-F9-L | 1.32 (1.21–1.62) | 2.49 (2.28–3.06) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rincón, M.; Calpena, A.C.; Fabrega, M.-J.; Garduño-Ramírez, M.L.; Espina, M.; Rodríguez-Lagunas, M.J.; García, M.L.; Abrego, G. Development of Pranoprofen Loaded Nanostructured Lipid Carriers to Improve Its Release and Therapeutic Efficacy in Skin Inflammatory Disorders. Nanomaterials 2018, 8, 1022. https://doi.org/10.3390/nano8121022
Rincón M, Calpena AC, Fabrega M-J, Garduño-Ramírez ML, Espina M, Rodríguez-Lagunas MJ, García ML, Abrego G. Development of Pranoprofen Loaded Nanostructured Lipid Carriers to Improve Its Release and Therapeutic Efficacy in Skin Inflammatory Disorders. Nanomaterials. 2018; 8(12):1022. https://doi.org/10.3390/nano8121022
Chicago/Turabian StyleRincón, María, Ana C. Calpena, María-José Fabrega, María L. Garduño-Ramírez, Marta Espina, María J. Rodríguez-Lagunas, María L. García, and Guadalupe Abrego. 2018. "Development of Pranoprofen Loaded Nanostructured Lipid Carriers to Improve Its Release and Therapeutic Efficacy in Skin Inflammatory Disorders" Nanomaterials 8, no. 12: 1022. https://doi.org/10.3390/nano8121022
APA StyleRincón, M., Calpena, A. C., Fabrega, M. -J., Garduño-Ramírez, M. L., Espina, M., Rodríguez-Lagunas, M. J., García, M. L., & Abrego, G. (2018). Development of Pranoprofen Loaded Nanostructured Lipid Carriers to Improve Its Release and Therapeutic Efficacy in Skin Inflammatory Disorders. Nanomaterials, 8(12), 1022. https://doi.org/10.3390/nano8121022