Up-Conversion Luminescence Properties of Lanthanide-Gold Hybrid Nanoparticles as Analyzed with Discrete Dipole Approximation
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Materials
2.2. Synthesis of NaYF4:18%Yb,2%Er
2.3. Synthesis of Core/Shell NaYF4:18%Yb,2%Er@NaYF4:30%Yb,10%Nd
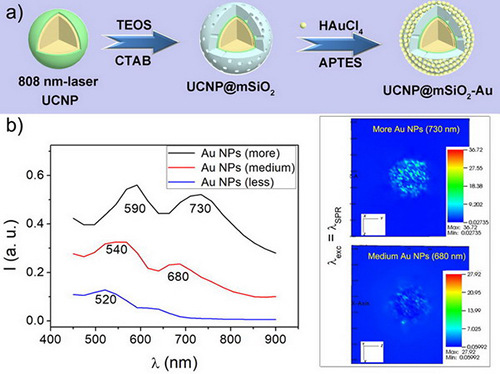
2.4. Synthesis of Mesoporous UCNP@mSiO2
2.5. Synthesis of Mesoporous UCNP@mSiO2-Au
2.6. Characterization
2.7. DDA Simulation
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dai, Y.; Xiao, H.; Liu, J.; Yuan, Q.; Ma, P.A.; Yang, D.; Li, C.; Cheng, Z.; Hou, Z.; Yang, P.; et al. In Vivo Multimodality Imaging and Cancer Therapy by Near-Infrared Light-Triggered trans-Platinum Pro-Drug-Conjugated Upconverison Nanoparticles. J. Am. Chem. Soc. 2013, 135, 18920–18929. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Yang, P.; Hu, B.; Xu, J.; Shang, W.; Tian, J. In Situ Growth Strategy to Integrate Up-Conversion Nanoparticles with Ultrasmall CuS for Photothermal Theranostics. ACS Nano 2017, 11, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Gnach, A.; Lipinski, T.; Bednarkiewicz, A.; Rybka, J.; Capobianco, J.A. Upconverting nanoparticles: Assessing the toxicity. Chem. Soc. Rev. 2015, 44, 1561–1584. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Roy, I.; Yang, C.; Prasad, P.N. Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy. Chem. Rev. 2016, 116, 2826–2885. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Feng, W.; Chang, J.; Tan, Y.-W.; Li, J.; Chen, M.; Sun, Y.; Li, F. Temperature-feedback upconversion nanocomposite for accurate photothermal therapy at facile temperature. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Deng, R.; MacDonald, M.A.; Chen, B.; Yuan, J.; Wang, F.; Chi, D.; Hor, T.S.A.; Zhang, P.; Liu, G.; et al. Enhancing multiphoton upconversion through energy clustering at sublattice level. Nat. Mater. 2014, 13, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.-C.; van Veggel, F.C.J.M. Absolute quantum yield measurements of colloidal NaYF4: Er3+, Yb3+ upconverting nanoparticles. Nanoscale 2010, 2, 1417–1419. [Google Scholar] [CrossRef] [PubMed]
- Komban, R.; Klare, J.P.; Voss, B.; Nordmann, J.; Steinhoff, H.-J.; Haase, M. An Electron Paramagnetic Resonance Spectroscopic Investigation on the Growth Mechanism of NaYF4:Gd Nanocrystals. Angew. Chem. Int. Ed. 2012, 51, 6506–6510. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, Q.; Feng, W.; Sun, Y.; Li, F. Upconversion Luminescent Materials: Advances and Applications. Chem. Rev. 2015, 115, 395–465. [Google Scholar] [PubMed]
- Yang, Y.; Liu, J.; Sun, X.; Feng, L.; Zhu, W.; Liu, Z.; Chen, M. Near-infrared light-activated cancer cell targeting and drug delivery with aptamer-modified nanostructures. Nano Res. 2016, 9, 139–148. [Google Scholar] [CrossRef]
- Liu, J.; Bu, W.; Pan, L.; Shi, J. NIR-Triggered Anticancer Drug Delivery by Upconverting Nanoparticles with Integrated Azobenzene-Modified Mesoporous Silica. Angew. Chem. Int. Ed. 2013, 52, 4375–4379. [Google Scholar] [CrossRef] [PubMed]
- Reineck, P.; Gibson, B.C. Near-Infrared Fluorescent Nanomaterials for Bioimaging and Sensing. Adv. Opt. Mater. 2017, 5. [Google Scholar] [CrossRef]
- Li, L.L.; Wu, P.W.; Hwang, K.; Lu, Y. An Exceptionally Simple Strategy for DNA-Functionalized Up-Conversion Nanoparticles as Biocompatible Agents for Nanoassembly, DNA Delivery, and Imaging. J. Am. Chem. Soc. 2013, 135, 2411–2414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, L.Y.; Pan, Y.W.; Zou, R.F.; Zhang, J.C.; Tian, Y.; Teng, Z.G.; Wang, S.J.; Ren, W.Z.; Xiao, X.S.; Zhang, J.C.; et al. 808 nm-excited upconversion nanoprobes with low heating effect for targeted magnetic resonance imaging and high-efficacy photodynamic therapy in HER2-overexpressed breast cancer. Biomaterials 2016, 103, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xue, B.; Kong, X.; Tu, L.; Liu, X.; Zhang, Y.; Chang, Y.; Luo, Y.; Zhao, H.; Zhang, H. 808 nm driven Nd3+-sensitized upconversion nanostructures for photodynamic therapy and simultaneous fluorescence imaging. Nanoscale 2015, 7, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Tian, G.; Gu, Z.; Yang, Y.; Gu, L.; Zhao, Y.; Ma, Y.; Yao, J. Elimination of Photon Quenching by a Transition Layer to Fabricate a Quenching-Shield Sandwich Structure for 800 nm Excited Upconversion Luminescence of Nd(3+) -Sensitized Nanoparticles. Adv. Mater. 2014, 26, 2831–2837. [Google Scholar] [CrossRef] [PubMed]
- Vetrone, F.; Naccache, R.; Mahalingam, V.; Morgan, C.G.; Capobianco, J.A. The Active-Core/Active-Shell Approach: A Strategy to Enhance the Upconversion Luminescence in Lanthanide-Doped Nanoparticles. Adv. Funct. Mater. 2009, 19, 2924–2929. [Google Scholar] [CrossRef]
- Bigall, N.C.; Parak, W.J.; Dorfs, D. Fluorescent, magnetic and plasmonic-Hybrid multifunctional colloidal nano objects. Nano Today 2012, 7, 282–296. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, C.; Feng, L.Z.; Yang, K.; Liu, Z. Functional Nanomaterials for Phototherapies of Cancer. Chem. Rev. 2014, 114, 10869–10939. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.A.; El-Sayed, M.A. Time dependence and signs of the shift of the surface plasmon resonance frequency in nanocages elucidate the nanocatalysis mechanism in hollow nanoparticles. Nano Lett. 2011, 11, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Skrabalak, S.E.; Chen, J.Y.; Sun, Y.G.; Lu, X.M.; Au, L.; Cobley, C.M.; Xia, Y.N. Gold Nanocages: Synthesis, Properties, and Applications. Acc. Chem. Res. 2008, 41, 1587–1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, M.A.; Qian, W.; El-Sayed, M.A. Following charge separation on the nanoscale in Cu(2)O-Au nanoframe hollow nanoparticles. Nano Lett. 2011, 11, 3285–3289. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Jiang, L.; Cai, F.H.; Wang, D.; He, S.L. Fluorescence-surface enhanced Raman scattering co-functionalized gold nanorods as near-infrared probes for purely optical in vivo imaging. Biomaterials 2011, 32, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-D.; Deng, Y.-L.; Li, C.-Y.; Lin, Y.; Tang, H.-W. Metal-enhanced fluorescent dye-doped silica nanoparticles and magnetic separation: A sensitive platform for one-step fluorescence detection of prostate specific antigen. Biosens. Bioelectron. 2017, 87, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.Z.; Shuhendler, A.J.; Ye, D.J.; Xu, J.J.; Chen, H.Y. Two-photon excitation nanoparticles for photodynamic therapyt. Chem. Soc. Rev. 2016, 45, 6725–6741. [Google Scholar] [CrossRef] [PubMed]
- Priyam, A.; Idris, N.M.; Zhang, Y. Gold nanoshell coated NaYF4 nanoparticles for simultaneously enhanced upconversion fluorescence and darkfield imaging. J. Mater. Chem. 2012, 22, 960–965. [Google Scholar] [CrossRef]
- Sudheendra, L.; Ortalan, V.; Dey, S.; Browning, N.D.; Kennedy, I.M. Plasmonic Enhanced Emissions from Cubic NaYF4:Yb:Er/Tm Nanophosphors. Chem. Mater. 2011, 23, 2987–2993. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Yang, P.; Dai, Y.; Gai, S.; He, F.; Lin, J. Lutecium Fluoride Hollow Mesoporous Spheres with Enhanced Up-Conversion Luminescent Bioimaging and Light-Triggered Drug Release by Gold Nanocrystals. ACS Appl. Mater. Interfaces 2014, 6, 15550–15563. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Yang, G.; Dai, Y.; Gai, S.; He, F.; Yang, P. Self-produced bubble-template synthesis of La2O3:Yb/Er@Au hollow spheres with markedly enhanced luminescence and release properties. Crystengcomm 2014, 16, 9612–9621. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Wang, Z.; Liu, X.; Xiong, Y. Modification of NaYF4:Yb,Er@SiO2 Nanoparticles with Gold Nanocrystals for Tunable Green-to-Red Upconversion Emissions. J. Phys. Chem. C 2011, 115, 3291–3296. [Google Scholar] [CrossRef]
- Ge, W.; Zhang, X.R.; Liu, M.; Lei, Z.W.; Knize, R.J.; Lu, Y. Distance Dependence of Gold-Enhanced Upconversion luminescence in Au/SiO2/Y2O3:Yb3+, Er3+ Nanoparticles. Theranostics 2013, 3, 282–288. [Google Scholar] [CrossRef] [PubMed]











© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, R.; Feng, M.; Parak, W.J. Up-Conversion Luminescence Properties of Lanthanide-Gold Hybrid Nanoparticles as Analyzed with Discrete Dipole Approximation. Nanomaterials 2018, 8, 989. https://doi.org/10.3390/nano8120989
Lv R, Feng M, Parak WJ. Up-Conversion Luminescence Properties of Lanthanide-Gold Hybrid Nanoparticles as Analyzed with Discrete Dipole Approximation. Nanomaterials. 2018; 8(12):989. https://doi.org/10.3390/nano8120989
Chicago/Turabian StyleLv, Ruichan, Miao Feng, and Wolfgang J. Parak. 2018. "Up-Conversion Luminescence Properties of Lanthanide-Gold Hybrid Nanoparticles as Analyzed with Discrete Dipole Approximation" Nanomaterials 8, no. 12: 989. https://doi.org/10.3390/nano8120989
APA StyleLv, R., Feng, M., & Parak, W. J. (2018). Up-Conversion Luminescence Properties of Lanthanide-Gold Hybrid Nanoparticles as Analyzed with Discrete Dipole Approximation. Nanomaterials, 8(12), 989. https://doi.org/10.3390/nano8120989