Synthesis of Graphite Oxide with Different Surface Oxygen Contents Assisted Microwave Radiation
Abstract
:1. Introduction
2. Results and Discussion
2.1. X-rays Diffraction
2.2. XPS and FTIR Spectroscopy
2.3. Raman Spectroscopy
2.4. Electron Microscopy
3. Materials and Methods
3.1. Materials and Reagents
3.2. Synthetic Procedures
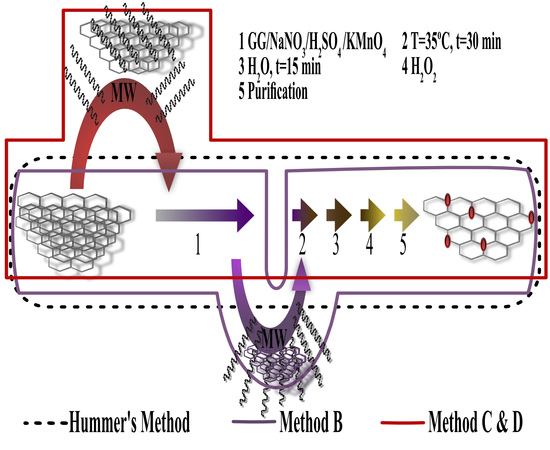
3.2.1. Method a Hummer’s Method
3.2.2. Method B
3.2.3. Method C
3.2.4. Method D
3.3. Characterization
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Muzyka, R.; Kwoka, M.; Smędowski, L.; Díez, N.; Gryglewicz, G. Oxidation of graphite by different modified Hummers methods. New Carbon Mater. 2017, 32, 15–20. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.-J. The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon 2013, 53, 38–49. [Google Scholar] [CrossRef]
- Ali Khan, Q.; Shaur, A.; Ali Khan, T.; Joya, Y.F.; Awan, M. Characterization of reduced graphene oxide produced through a modified Hoffman method. Cogent Chem. 2017, 3, 1–9. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J.; Kim, K.S.K.S.; Zhao, Y.; et al. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, U.; König, E. Untersuchungen über Graphitoxyd. Z. Anorg. Allg. Chem. 1937, 234, 311–336. [Google Scholar] [CrossRef]
- Brodie, B.C. On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar] [CrossRef]
- Guerrero, C.; Sepulveda, S.; Cruz, R. Enzymatic synthesis of polyaniline/graphite oxide nanocomposites. MRS Proc. 2012, 1448. [Google Scholar] [CrossRef]
- Voiry, D.; Yang, J.; Kupferberg, J.; Fullon, R.; Lee, C.; Jeong, H.Y.; Shin, H.S.; Chhowalla, M. High-quality graphene via microwave reduction of solution-exfoliated graphene oxide. Science 2016, 353, 1413–1417. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; An, J.; Potts, J.R.; Velamakanni, A.; Murali, S.; Ruoff, R.S. Hydrazine-reduction of graphite-and graphene oxide. Carbon 2011, 49, 3019–3023. [Google Scholar] [CrossRef]
- Zhao, N.; Wen, C.-Y.; Zhang, W.; Wu, D.-P.; Zhang, Z.-B.; Zhang, S.-L. Liquid-phase and solid-phase microwave irradiations for reduction of graphite oxide. Chin. Phys. B 2014, 23, 128101. [Google Scholar] [CrossRef]
- Xu, Z.; Li, H.; Li, W.; Cao, G.; Zhang, Q.; Li, K.; Fu, Q.; Wang, J. Large-scale production of graphene by microwave synthesis and rapid cooling. Chem. Commun. 2011, 47, 1166–1168. [Google Scholar] [CrossRef] [PubMed]
- Menéndez, J.A.; Arenillas, A.; Fidalgo, B.; Fernández, Y.; Zubizarreta, L.; Calvo, E.G.; Bermúdez, J.M. Microwave heating processes involving carbon materials. Fuel Process. Technol. 2010, 91, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Kumar, R.; Singh, D.P. Graphene oxide: Strategies for synthesis, reduction and frontier applications. RSC Adv. 2016, 6, 64993–65011. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, Z.; Zhou, Q.; Gogotsi, Y.; Qiu, J. The role of microwave absorption on formation of graphene from graphite oxide. Carbon 2012, 50, 3267–3273. [Google Scholar] [CrossRef]
- Hassan, M.A.H.; Abdelsayed, V.; Khder, A.E.R.S.; AbouZeid, K.M.; Terner, J.; El-Shall, M.S.; Al-Resayes, S.I.; El-Azhary, A.A. Microwave synthesis of graphene sheets supporting metal nanocrystals in aqueous and organic media. J. Mater. Chem. 2009, 19, 3832–3837. [Google Scholar] [CrossRef]
- Garino, N.; Sacco, A.; Castellino, M.; Muñ Oz-Tabares, J.; Chiodoni, A.; Agostino, V.; Margaria, V.; Gerosa, M.; Massaglia, G.; Quaglio, M. Microwave-assisted synthesis of reduced graphene oxide/sno2 nanocomposite for oxygen reduction reaction in microbial fuel cells. ACS Appl. Mater. Interfaces 2016, 8, 4633–4643. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Li, X.; Wang, H.; Zhang, L. Microwave assisted synthesis of reduced graphene oxide incorporated MOF-derived ZnO composites for photocatalytic application. Catal. Commun. 2017, 66, 5–88. [Google Scholar] [CrossRef]
- Kim, Y.; Cho, E.-S.; Park, S.-J.; Kim, S. One-pot microwave-assisted synthesis of reduced graphene oxide/nickel cobalt double hydroxide composites and their electrochemical behavior. J. Ind. Eng. Chem. 2016, 33, 108–114. [Google Scholar] [CrossRef]
- Chaban, V.V.; Prezhdo, O.V. Microwave reduction of graphene oxide rationalized by reactive molecular dynamics. Nanoscale 2017, 9, 4024–4033. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Velamakanni, A.; Piner, R.D.; Ruoff, R.S. Microwave assisted exfoliation and reduction of graphite oxide for ultracapacitors. Carbon 2010, 48, 2118–2122. [Google Scholar] [CrossRef]
- Han, H.; Chen, Y.; Wang, Z. Effect of microwave irradiation on reduction of graphene oxide films. RSC Adv. 2015, 92940–92946. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Z.-R.; Fu, X.; Xu, Y.-J. TiO2-Graphene nanocomposites for gas-phase photocatalytic degradation of volatile aromatic pollutant: Is TiO2-Graphene Truly Different from Other TiO2-Carbon Composite Materials? ACS Nano 2010, 4, 7303–7314. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Li, M.; Zhi, M.; Manivannan, A.; Wu, N. Reduced graphene oxide/titanium dioxide composites for supercapacitor electrodes: Shape and coupling effects. J. Mater. Chem. 2012, 22, 19161–19167. [Google Scholar] [CrossRef]
- Wang, L.; Huang, Y.; Sun, X.; Huang, H.; Liu, P.; Zong, M.; Wang, Y. Synthesis and microwave absorption enhancement of graphene@Fe3O4@SiO2@NiO nanosheet hierarchical structures. Nanoscale 2014, 6, 3157–3164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, H.; Shen, G.; Cheng, P.; Zhang, J.; Guo, S. Reduction of graphene oxide via L-ascorbic acid. Chem. Commun. 2010, 46, 1112–1114. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Kim, T.; Choi, J.; Park, J.; Kim, Y.S.; Chang, M.S.; Jung, H.; Park, K.T.; Yang, S.J.; Park, C.R. Hidden second oxidation step of Hummers method. Chem. Mater. 2016, 28, 756–764. [Google Scholar] [CrossRef]
- Dimiev, A.M.; Tour, J.M. Mechanism of graphene oxide formation. ACS Nano 2014, 8, 3060–3068. [Google Scholar] [CrossRef] [PubMed]
- Botas, C.; Álvarez, P.; Blanco, P.; Granda, M.; Blanco, C.; Santamaría, R.; Romasanta, L.J.; Verdejo, R.; López-Manchado, M.A.; Menéndez, R. Graphene materials with different structures prepared from the same graphite by the Hummers and Brodie methods. Carbon 2013, 65, 156–164. [Google Scholar] [CrossRef]
- Vaks, V.L.; Domraehev, G.A.; Rodygin, Y.L.; Selivanovskii, D.A.; Spivak, E.I. Dissociation of water by microwave radiation. Radiophys. Quantum Electron. 1994, 37, 149–154. [Google Scholar] [CrossRef]
- Kumar, D.; Raghavan, C.M.; Sridhar, C.; Shin, J.-H.; Ryu, S.H.; Jang, K.; Shin, D.-S. Microwave-assisted synthesis, characterization of reduced graphene oxide, and its antibacterial activity. Bull. Korean Chem. Soc. 2015, 36, 2034–2038. [Google Scholar] [CrossRef]
- Dash, S.; Patel, S.; Mishra, B.K. Oxidation by permanganate: Synthetic and mechanistic aspects. Tetrahedron 2008, 65, 707–739. [Google Scholar] [CrossRef]
- Drummond, A.Y.; Waters, W.A. Stages in oxidations of organic compounds by potassium per-manganate. Part I. The Permanganate-Manganate stage. Part II. The manganic-manganous stage. J. Chem. Soc. 1953, 435–443. [Google Scholar] [CrossRef]
- Rigby, W. Hydroxylations with potassium manganate. J. Chem. Soc. 1956, 2452–2454. [Google Scholar] [CrossRef]
- Waters, W.A. Mechanisms of oxidation by compounds of chromium and manganese. Q. Rev. Chem. Soc. 1958, 12, 277–300. [Google Scholar] [CrossRef]
- Kovtyukhova, N.I. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 1999, 11, 771–778. [Google Scholar] [CrossRef]
- Parvez, K.; Wu, Z.; Li, R.; Liu, X.; Graf, R. Exfoliation of graphite into graphene in aqueous solutions. J. Am. Chem. Soc. 2014, 136, 6083–6091. [Google Scholar] [CrossRef] [PubMed]
- Royer, D.J. Evidence for the existence of the ion in sulphuric acid solutions of potassium permanganate. J. Inorg. Nucl. Chem. 1961, 17, 159–167. [Google Scholar] [CrossRef]
- Symons, M.C.R. The mechanism of decomposition of potassium permanganate in alkaline solution. Part II. *The use of water enriched in 18O as solvent. J. Chem. Soc. 1954, 3676–3679. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yan, L.; Bangal, P.R. Preparation of graphene by the rapid and mild thermal reduction of graphene oxide induced by microwaves. Carbon 2009, 48, 1146–1152. [Google Scholar] [CrossRef]
- Wong, C.H.A.; Jankovský, O.; Sofer, Z.; Pumera, M. Vacuum-assisted microwave reduction/exfoliation of graphite oxide and the influence of precursor graphite oxide. Carbon 2014, 77, 508–517. [Google Scholar] [CrossRef]
- Shim, S.H.; Kim, K.T.; Lee, J.U.; Jo, W.H. Facile method to functionalize graphene oxide and its application to poly(ethylene terephthalate)/graphene composite. ACS Appl. Mater. Interfaces 2012, 4, 4184–4191. [Google Scholar] [CrossRef] [PubMed]
- Acik, M.; Lee, G.; Mattevi, C.; Pirkle, A.; Wallace, R.M.; Chhowalla, M.; Cho, K.; Chabal, Y. The role of oxygen during thermal reduction of graphene oxide studied by infrared absorption spectroscopy. J. Phys. Chem. C 2011, 115, 19761–19781. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Huang, L.; Li, C.; Shi, G. High-yield preparation of graphene oxide from small graphite flakes via an improved Hummers method with a simple purification process. Carbon 2015, 81, 826–834. [Google Scholar] [CrossRef]
- Guerrero-Contreras, J. Graphene oxide powders with different oxidation degree, prepared by synthesis variations of the Hummers method. Mater. Chem. Phys. 2015, 153, 209–220. [Google Scholar] [CrossRef]
- Talazin, A.; Mercier, G.; Klechikov, A.; Hedenström, M.; Johnels, D.; Wei, D.; Cotton, D.; Opitz, A.; Monns, E. Brodie vs. Hummers graphite oxides for preparation of multilayed materials. Carbon 2017, 115, 430–440. [Google Scholar] [CrossRef]
- Gnana Kumar, G.; Justice Babu, K.; Nahm, K.S.; Hwang, Y.J. A facile one-pot green synthesis of reduced graphene oxide and its composites for non-enzymatic hydrogen peroxide sensor applications. RSC Adv. 2014, 4, 7944–7951. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.B.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Dao, T.D.; Jeong, H.M. Graphene prepared by thermal reduction–exfoliation of graphite oxide: Effect of raw graphite particle size on the properties of graphite oxide and graphene. Mater. Res. Bull. 2015, 70, 651–657. [Google Scholar] [CrossRef]
- Celiešiūte, R.; Trusovas, R.; Niaura, G.; Švedas, V.; Račiukaitis, G.; Ruzele, Z.; Pauliukaite, R. Influence of the laser irradiation on the electrochemical and spectroscopic peculiarities of graphene–chitosan composite film. Electrochim. Acta 2014, 132, 265–276. [Google Scholar] [CrossRef]
- Kaniyoor, A.; Ramaprabhu, S. A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv. 2012, 2, 032183-1–032183-13. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Park, J.S.; Reina, A.; Saito, R.; Kong, J.; Dresselhaus, G.; Dresselhaus, M.S. G’ band Raman spectra of single, double and triple layer graphene. Carbon 2009, 47, 1303–1310. [Google Scholar] [CrossRef]
- Matsumoto, M.; Saito, Y.; Park, C.; Fukushima, T.; Aida, T. Ultrahigh-throughput exfoliation of graphite into pristine single-layer graphene using microwaves and molecularly engineered ionic liquids. Nat. Chem. 2015, 7, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Zhuangjun, F.; Guilian, L.; Chao, Z.; Dashou, X. A rapid and efficient method to prepare exfoliated graphite by microwave irradiation. Carbon 2008, 47, 313–347. [Google Scholar] [CrossRef]
- Drewniak, S.; Pustelny, T.; Muzyka, R.; Konieczny, G.; Kałużyński, P. The effect of oxidation and reduction processes of graphite on physicochemical properties of graphite oxide and reduced graphene oxide. Photonics Lett. Pol. 2014, 6, 130–132. [Google Scholar] [CrossRef]
- Hao, J.; Liao, Y.; Zhong, Y.; Shu, D.; He, C.; Guo, S.; Huang, Y.; Zhong, J.; Hu, L. Three-dimensional graphene layers prepared by a gas-foaming method for supercapacitor applications. Carbon 2015, 94, 879–887. [Google Scholar] [CrossRef]
- Khenfouch, M.; Buttner, U.; Baïtoul, M.; Maaza, M. Synthesis and characterization of mass produced high quality few layered graphene sheets via a chemical method. Graphene 2014, 7–13. [Google Scholar] [CrossRef]
- Lling-Lling, T.; Wee-Jun, O.; Siang-Piao, C.; Abdul Rahman, M. Reduced graphene oxide-TiO2 nanocomposite as a promising visible-light-active photocatalyst for conversion of carbon dioxide. Nanoscale Res. Lett. 2013, 8, 1–9. [Google Scholar] [CrossRef]








| Sample | Temperature °C | Time (min) |
|---|---|---|
| B1 | 60 | 5 |
| B2 | 60 | 20 |
| B3 | 80 | 20 |
| Sample | Temperature °C | Time (min) |
|---|---|---|
| C1 | 60 | 5 |
| C2 | 60 | 20 |
| Sample | Temperature °C | Time (min) |
|---|---|---|
| D1 | 60 | 5 |
| D2 | 60 | 20 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibarra-Hernández, A.; Vega-Rios, A.; Osuna, V. Synthesis of Graphite Oxide with Different Surface Oxygen Contents Assisted Microwave Radiation. Nanomaterials 2018, 8, 106. https://doi.org/10.3390/nano8020106
Ibarra-Hernández A, Vega-Rios A, Osuna V. Synthesis of Graphite Oxide with Different Surface Oxygen Contents Assisted Microwave Radiation. Nanomaterials. 2018; 8(2):106. https://doi.org/10.3390/nano8020106
Chicago/Turabian StyleIbarra-Hernández, Adriana, Alejandro Vega-Rios, and Velia Osuna. 2018. "Synthesis of Graphite Oxide with Different Surface Oxygen Contents Assisted Microwave Radiation" Nanomaterials 8, no. 2: 106. https://doi.org/10.3390/nano8020106
APA StyleIbarra-Hernández, A., Vega-Rios, A., & Osuna, V. (2018). Synthesis of Graphite Oxide with Different Surface Oxygen Contents Assisted Microwave Radiation. Nanomaterials, 8(2), 106. https://doi.org/10.3390/nano8020106