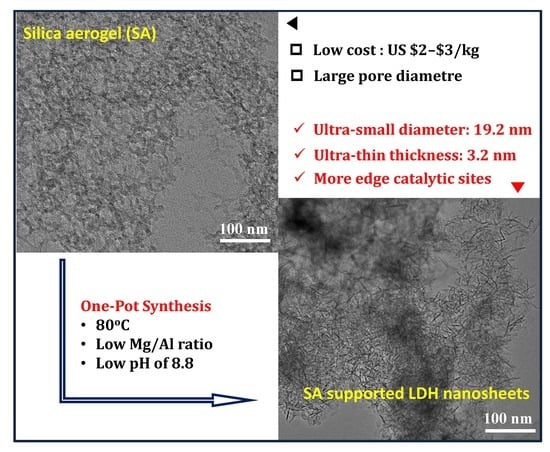
Small-Sized Mg–Al LDH Nanosheets Supported on Silica Aerogel with Large Pore Channels: Textural Properties and Basic Catalytic Performance after Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental
2.3. Characterization
2.4. Catalytic Evaluation
3. Results and Discussion

4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lv, W.; Mei, Q.; Fu, H.; Xiao, J.; Du, M.; Zheng, Q. A general strategy for the synthesis of layered double hydroxide nanoscrolls on arbitrary substrates: Its formation and multifunction. J. Mater. Chem. A 2017, 5, 19079–19090. [Google Scholar] [CrossRef]
- Nishimura, S.; Takagaki, A.; Ebitani, K. Characterization, synthesis and catalysis of hydrotalcite-related materials for highly efficient materials transformations. Green Chem. 2013, 15, 2026–2042. [Google Scholar] [CrossRef]
- Li, C.M.; Wei, M.; Evans, D.G.; Duan, X. Layered Double Hydroxide-based Nanomaterials as Highly Efficient Catalysts and Adsorbents. Small 2014, 10, 4469–4486. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; O’Hare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Sideris, P.J.; Nielsen, U.G.; Gan, Z.H.; Grey, C.P. Mg/Al ordering in layered double hydroxides revealed by multinuclear NMR spectroscopy. Science 2008, 321, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Ma, R.Z.; Ebina, Y.; Iyi, N.; Sasaki, T. Positively charged nanosheets derived via total delamination of layered double hydroxides. Chem. Mater. 2005, 17, 4386–4391. [Google Scholar] [CrossRef]
- Takehira, K.; Shishido, T.; Wang, P.; Kosaka, T.; Takaki, K. Autothermal reforming of CH4 over supported Ni catalysts prepared from Mg-Al hydrotalcite-like anionic clay. J. Catal. 2004, 221, 43–54. [Google Scholar] [CrossRef]
- Kantam, M.L.; Choudary, B.M.; Reddy, C.V.; Rao, K.K.; Figueras, F. Aldol and Knoevenagel condensations catalysed by modified Mg-Al hydrotalcite: A solid base as catalyst useful in synthetic organic chemistry. Chem. Commun. 1998, 1033–1034. [Google Scholar] [CrossRef]
- Liu, Y.; Lotero, E.; Goodwin, J.G.; Mo, X. Transesterification of poultry fat with methanol using Mg-A1 hydrotalcite derived catalysts. Appl. Catal. A-Gen. 2007, 331, 138–148. [Google Scholar] [CrossRef]
- Fan, G.L.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef] [PubMed]
- Shumaker, J.L.; Crofcheck, C.; Tackett, S.A.; Santillan-Jimenez, E.; Morgan, T.; Ji, Y.; Crocker, M.; Toops, T.J. Biodiesel synthesis using calcined layered double hydroxide catalysts. Appl. Catal. B-Environ. 2008, 82, 120–130. [Google Scholar] [CrossRef]
- Reyes, I.C.; Salmones, J.; Zeifert, B.; Contreras, J.L.; Rojas, F. Transesterification of canola oil catalized by calcined Mg-Al hydrotalcite doped with nitratine. Chem. Eng. Sci. 2014, 119, 174–181. [Google Scholar] [CrossRef]
- Delidovich, I.; Palkovits, R. Structure-performance correlations of Mg-Al hydrotalcite catalysts for the isomerization of glucose into fructose. J. Catal. 2015, 327, 1–9. [Google Scholar] [CrossRef]
- Yu, S.; Kim, E.; Park, S.; Song, I.K.; Jung, J.C. Isomerization of glucose into fructose over Mg-Al hydrotalcite catalysts. Catal. Commun. 2012, 29, 63–67. [Google Scholar] [CrossRef]
- Lee, G.; Jeong, Y.; Takagaki, A.; Jung, J.C. Sonication assisted rehydration of hydrotalcite catalyst for isomerization of glucose to fructose. J. Mol. Catal. A-Chem. 2014, 393, 289–295. [Google Scholar] [CrossRef]
- Bharali, D.; Devi, R.; Bharali, P.; Deka, R.C. Synthesis of high surface area mixed metal oxide from the NiMgAl LDH precursor for nitro-aldol condensation reaction. New J. Chem. 2015, 39, 172–178. [Google Scholar] [CrossRef]
- Kikhtyanin, O.; Lesnik, E.; Kubicka, D. The occurrence of Cannizzaro reaction over Mg-Al hydrotalcites. Appl. Catal. A-Gen. 2016, 525, 215–225. [Google Scholar] [CrossRef]
- Angelescu, E.; Pavel, O.D.; Birjega, R.; Florea, M.; Zavoianu, R. The impact of the “memory effect” on the catalytic activity of Mg/Al; Mg,Zn/Al; Mg/Al,Ga hydrotalcite-like compounds used as catalysts for cycloxene epoxidation. Appl. Catal. A-Gen. 2008, 341, 50–57. [Google Scholar] [CrossRef]
- Wu, G.D.; Wang, X.L.; Chen, B.; Li, J.P.; Zhao, N.; Wei, W.; Sun, Y.H. Fluorine-modified mesoporous Mg-Al mixed oxides: Mild and stable base catalysts for O-methylation of phenol with dimethyl carbonate. Appl. Catal. A-Gen. 2007, 329, 106–111. [Google Scholar] [CrossRef]
- Kikhtyanin, O.; Hora, L.; Kubicka, D. Unprecedented selectivities in aldol condensation over Mg-Al hydrotalcite in a fixed bed reactor setup. Catal. Commun. 2015, 58, 89–92. [Google Scholar] [CrossRef]
- Oka, Y.; Kuroda, Y.; Matsuno, T.; Kamata, K.; Wada, H.; Shimojima, A.; Kuroda, K. Preparation of Mesoporous Basic Oxides through Assembly of Monodispersed Mg-Al Layered Double Hydroxide Nanoparticles. Chem.-Eur. J. 2017, 23, 9362–9368. [Google Scholar] [CrossRef] [PubMed]
- Hora, L.; Kelbichova, V.; Kikhtyanin, O.; Bortnovskiy, O.; Kubicka, D. Aldol condensation of furfural and acetone over Mg-Al layered double hydroxides and mixed oxides. Catal. Today 2014, 223, 138–147. [Google Scholar] [CrossRef]
- Roelofs, J.C.A.A.; Lensveld, D.J.; van Dillen, A.J.; de Jong, K.P. On the Structure of Activated Hydrotalcites as Solid Base Catalysts for Liquid-Phase Aldol Condensation. J. Catal. 2001, 203, 184–191. [Google Scholar] [CrossRef]
- Tichit, D.; Gérardin, C.; Durand, R.; Coq, B. Layered double hydroxides: Precursors for multifunctional catalysts. Top. Catal. 2006, 39, 89–96. [Google Scholar] [CrossRef]
- Abelló, S.; Medina, F.; Tichit, D.; Pérez-Ramírez, J.; Groen, J.C.; Sueiras, J.E.; Salagre, P.; Cesteros, Y. Aldol Condensations Over Reconstructed Mg–Al Hydrotalcites: Structure–Activity Relationships Related to the Rehydration Method. Chem.-Eur. J. 2005, 11, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Abello, S.; Medina, F.; Tichit, D.; Perez-Ramirez, J.; Cesteros, Y.; Salagre, P.; Sueiras, J.E. Nanoplatelet-based reconstructed hydrotalcites: Towards more efficient solid base catalysts in aldol condensations. Chem. Commun. 2005, 1453–1455. [Google Scholar] [CrossRef] [PubMed]
- Winter, F.; van Dillen, A.J.; de Jong, K.P. Supported hydrotalcites as highly active solid base catalysts. Chem. Commun. 2005, 3977–3979. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shi, J. In situ assembly of layered double hydroxide nano-crystallites within silica mesopores and its high solid base catalytic activity. Chem. Commun. 2008, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, M.; Wang, Q.; Buffet, J.-C.; O’Hare, D. Synthesis and characterisation of aqueous miscible organic-layered double hydroxides. J. Mater. Chem. A 2014, 2, 15102–15110. [Google Scholar] [CrossRef]
- Chen, C.; Felton, R.; Buffet, J.-C.; O’Hare, D. Core-shell SiO2@LDHs with tuneable size, composition and morphology. Chem. Commun. 2015, 51, 3462–3465. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wangriya, A.; Buffet, J.-C.; O’Hare, D. Tuneable ultra high specific surface area Mg/Al-CO3 layered double hydroxides. Dalton Trans. 2015, 44, 16392–16398. [Google Scholar] [CrossRef] [PubMed]
- Creasey, J.J.; Parlett, C.M.A.; Manayil, J.C.; Isaacs, M.A.; Wilson, K.; Lee, A.F. Facile route to conformal hydrotalcite coatings over complex architectures: A hierarchically ordered nanoporous base catalyst for FAME production. Green Chem. 2015, 17, 2398–2405. [Google Scholar] [CrossRef]
- Garcia-Gallastegui, A.; Iruretagoyena, D.; Mokhtar, M.; Asiri, A.M.; Basahel, S.N.; Al-Thabaiti, S.A.; Alyoubi, A.O.; Chadwick, D.; Shaffer, M.S.P. Layered double hydroxides supported on multi-walled carbon nanotubes: Preparation and CO2 adsorption characteristics. J. Mater. Chem. 2012, 22, 13932–13940. [Google Scholar] [CrossRef]
- Garcia-Gallastegui, A.; Iruretagoyena, D.; Gouvea, V.; Mokhtar, M.; Asiri, A.M.; Basahel, S.N.; Al-Thabaiti, S.A.; Alyoubi, A.O.; Chadwick, D.; Shaffer, M.S.P. Graphene Oxide as Support for Layered Double Hydroxides: Enhancing the CO2 Adsorption Capacity. Chem. Mater. 2012, 24, 4531–4539. [Google Scholar] [CrossRef]
- Memon, J.; Sun, J.; Meng, D.; Ouyang, W.; Memon, M.A.; Huang, Y.; Yan, S.; Geng, J. Synthesis of graphene/Ni-Al layered double hydroxide nanowires and their application as an electrode material for supercapacitors. J. Mater. Chem. A 2014, 2, 5060–5067. [Google Scholar] [CrossRef]
- Chang, Y.-P.; Chen, Y.-C.; Chang, P.-H.; Chen, S.-Y. Synthesis, Characterization, and CO2 Adsorptive Behavior of Mesoporous AlOOH-Supported Layered Hydroxides. ChemSusChem 2012, 5, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Byles, C.F.H.; Buffet, J.-C.; Rees, N.H.; Wu, Y.; O’Hare, D. Core-shell zeolite@aqueous miscible organic-layered double hydroxides. Chem. Sci. 2016, 7, 1457–1461. [Google Scholar] [CrossRef]
- Xu, J.; Ma, C.; Cao, J.; Chen, Z. Facile synthesis of core-shell nanostructured hollow carbon nanospheres@nickel cobalt double hydroxides as high-performance electrode materials for supercapacitors. Dalton Trans. 2017, 46, 3276–3283. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; He, F.; Gai, S.; Zhang, S.; Li, L.; Yang, P. Nitrogen-enriched, double-shelled carbon/layered double hydroxide hollow microspheres for excellent electrochemical performance. Nanoscale 2014, 6, 10887–10895. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Li, J.; Zhang, H. Hierarchical hollow nanostructured core@shell recyclable catalysts [gamma]-Fe2O3@LDH@Au25-x for highly efficient alcohol oxidation. Green Chem. 2016, 18, 5900–5914. [Google Scholar] [CrossRef]
- Shirotori, M.; Nishimura, S.; Ebitani, K. Fine-crystallized LDHs prepared with SiO2 spheres as highly active solid base catalysts. J. Mater. Chem. A 2017, 5, 6947–6957. [Google Scholar] [CrossRef]
- Kwok, W.L.J.; Crivoi, D.-G.; Chen, C.; Buffet, J.-C.; O’Hare, D. Silica@layered double hydroxide core-shell hybrid materials. Dalton Trans. 2018, 47, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wang, P.; Lim, T.-T.; Liu, L.; Liu, S.; Xu, R. A facile synthesis of monodispersed hierarchical layered double hydroxide on silica spheres for efficient removal of pharmaceuticals from water. J. Mater. Chem. A 2013, 1, 3877–3880. [Google Scholar] [CrossRef]
- Buffet, J.-C.; Byles, C.F.H.; Felton, R.; Chen, C.; O’Hare, D. Metallocene supported core@LDH catalysts for slurry phase ethylene polymerisation. Chem. Commun. 2016, 52, 4076–4079. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yee, L.K.; Gong, H.; Zhang, Y.; Xu, R. A facile synthesis of strong near infrared fluorescent layered double hydroxide nanovehicles with an anticancer drug for tumor optical imaging and therapy. Nanoscale 2013, 5, 4314–4320. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, K.M.; Molgero Westrup, K.C.; da Silva Junior, R.M.; Jaerger, S.; Wypych, F.; Nakagaki, S. Oxidation catalyst obtained by the immobilization of layered double hydroxide/Mn(iii) porphyrin on monodispersed silica spheres. Dalton Trans. 2018. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liu, S.; Liu, Y.; Gu, Y.; Zeng, G.; Cai, X.; Yan, Z.; Yang, C.; Hu, X.; Chen, B. One-pot synthesis of carbon supported calcined-Mg/Al layered double hydroxides for antibiotic removal by slow pyrolysis of biomass waste. Sci. Rep. 2016, 6, 39691. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Zhang, H.; Fan, T.; Chen, J.; Duan, X. Nearly monodispersed core-shell structural Fe3O4@DFUR-LDH submicro particles for magnetically controlled drug delivery and release. Chem. Commun. 2011, 47, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Mayadevi, S. Cellulose supported layered double hydroxides for the adsorption of fluoride from aqueous solution. Chemosphere 2008, 72, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhou, H.; Sun, J.; Qin, F.; Yu, F.; Bao, J.; Yu, Y.; Chen, S.; Ren, Z. Cu nanowires shelled with NiFe layered double hydroxide nanosheets as bifunctional electrocatalysts for overall water splitting. Energy Environ. Sci. 2017, 10, 1820–1827. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, D.; Zou, K.; He, J.; Duan, X. A novel core-shell structured magnetic organic-inorganic nanohybrid involving drug-intercalated layered double hydroxides coated on a magnesium ferrite core for magnetically controlled drug release. J. Mater. Chem. 2009, 19, 3069–3077. [Google Scholar] [CrossRef]
- Soleimani Dorcheh, A.; Abbasi, M.H. Silica aerogel; synthesis, properties and characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; Portugal, A. An overview on silica aerogels synthesis and different mechanical reinforcing strategies. J. Non-Cryst. Solids 2014, 385, 55–74. [Google Scholar] [CrossRef]
- Xia, K.; Lang, W.-Z.; Li, P.-P.; Long, L.-L.; Yan, X.; Guo, Y.-J. The influences of Mg/Al molar ratio on the properties of PtIn/Mg(Al)O-x catalysts for propane dehydrogenation reaction. Chem. Eng. J. 2016, 284, 1068–1079. [Google Scholar] [CrossRef]
- Du, X.; Zhang, D.; Shi, L.; Gao, R.; Zhang, J. Coke-and sintering-resistant monolithic catalysts derived from in situ supported hydrotalcite-like films on Al wires for dry reforming of methane. Nanoscale 2013, 5, 2659–2663. [Google Scholar] [CrossRef] [PubMed]
- Tomar, R.; Singh, N.; Rathee, G.; Kumar, N.; Tomar, V.; Chandra, R. Synthesis and Characterization of Hybrid Mg(OH)(2) and CeCO3OH Composite with Improved Activity Towards Henry Reaction. Asian J. Org. Chem. 2017, 6, 1728–1732. [Google Scholar] [CrossRef]
- Feng, X.; Wang, L.; Yao, X.; Dong, H.; Wang, X.; Wang, Y. Trace water/amino-modified silica aerogel catalytic system in the one-pot sequential reaction of benzaldehyde dimethyl acetal and nitromethane. Catal. Commun. 2017, 90, 106–110. [Google Scholar] [CrossRef]
- Tang, Y.; Gu, X.F.; Meng, M.; Xu, J.F. Direct Henry reactions with modified calcium oxide as solid catalyst. Res. Chem. Intermed. 2013, 39, 3715–3725. [Google Scholar] [CrossRef]
- Akutu, K.; Kabashima, H.; Seki, T.; Hattori, H. Nitroaldol reaction over solid base catalysts. Appl. Catal. A-Gen. 2003, 247, 65–74. [Google Scholar] [CrossRef]
- Choudary, B.M.; Kantam, M.L.; Reddy, C.V.; Rao, K.K.; Figueras, F. Henry reactions catalysed by modified Mg-Al hydrotalcite: An efficient reusable solid base for selective synthesis of beta-nitroalkanols. Green Chem. 1999, 1, 187–189. [Google Scholar] [CrossRef]
- Huang, J.P.; Li, C.M.; Tao, L.L.; Zhu, H.L.; Hu, G. Synthesis, characterization and heterogeneous base catalysis of amino functionalized lanthanide metal-organic frameworks. J. Mol. Struct. 2017, 1146, 853–860. [Google Scholar] [CrossRef]
- Komura, K.; Kawamura, T.; Sugi, Y. Layered silicate PLS-1: A new solid base catalyst for C-C bond forming reactions. Catal. Commun. 2007, 8, 644–648. [Google Scholar] [CrossRef]
- Motokura, K.; Viswanadham, N.; Dhar, G.M.; Iwasawa, Y. Creation of acid-base bifunctional catalysis for efficient C-C coupling reactions by amines immobilization on SiO2, silica-alumina, and nano-H-ZSM-5. Catal. Today 2009, 141, 19–24. [Google Scholar] [CrossRef]
- He, Y.X.; Jawad, A.; Li, X.; Atanga, M.; Rezaei, F.; Rownaghi, A.A. Direct aldol and nitroaldol condensation in an aminosilane-grafted Si/Zr/Ti composite hollow fiber as a heterogeneous catalyst and continuous-flow reactor. J. Catal. 2016, 341, 149–159. [Google Scholar] [CrossRef]
- Shaikh, M.; Sahu, M.; Gavel, P.K.; Turpu, G.R.; Khilari, S.; Pradhan, D.; Ranganath, K.V.S. Mg-NHC complex on the surface of nanomagnesium oxide for catalytic application. Catal. Commun. 2016, 84, 89–92. [Google Scholar] [CrossRef]
- Thangaraj, B.; Jayaraj, C.; Srinivasan, R.; Ayyamperumal, S. Diaminosilane-functionalized on silicate-stabilised hydrotalcite (MA-HTSi-DA): As potential catalyst for nitro-aldol condensation. J. Mol. Catal. A-Chem. 2015, 409, 11–18. [Google Scholar] [CrossRef]






| Samples | SBET (m2·g) | Vp (cm3·g) | Dp (nm) | DLDH a (nm) | THLDH b (nm) | Mg/Al Ratio | Mg/Al/Si Ratio |
|---|---|---|---|---|---|---|---|
| SA/LDH-Mg2Al-80 | 587.4 | 1.30 | 11.9 | 19.2 | 3.2 | 1.24 | 0.58:0.46:1 |
| SA/LDH-Mg2Al-105 | 460.5 | 0.90 | 9.3 | 53.0 | 4.5 | 1.46 | 0.69:0.47:1 |
| SA/LDH-Mg2Al-150 | 429.5 | 0.96 | 9.0 | 57.4 | 4.3 | 1.51 | 0.77:0.51:1 |
| SA/LDH-Mg5Al2-80 | 456.0 | 0.98 | 9.9 | 31.4 | 3.2 | 2.10 | 0.83:0.39:1 |
| SA/LDH-Mg3Al-80 | 496.1 | 1.12 | 9.2 | 35.8 | 3.6 | 2.27 | 0.78:0.34:1 |
| SA | 803.8 | 5.92 | 34.4 | — | — | — | — |
| LDH-Mg2Al-80 | 120.6 | 0.69 | 21.2 | 87.1 | <20 | 1.94 | — |
| Samples | CO2-TPD Peak Position (°C) | Total Peak Area (a.u.) a | ||
|---|---|---|---|---|
| I | II | III | ||
| SA/LDH-Mg2Al-80 | 160.6 | 455.6 | 649.3 | 86188.7 |
| SA/LDH-Mg2Al-105 | 131.3 | 430.6 | 612.0 | 68865.8 |
| SA/LDH-Mg2Al-150 | 142.9 | 417.6 | 605.7 | 43227.1 |
| SA/LDH-Mg5Al2-80 | 140.2 | 431.8 | 643.1 | 92283.9 |
| SA/LDH-Mg3Al-80 | 143.1 | 458.5 | 628.9 | 74727.8 |
| LDH-Mg2Al-80 | 121.5 | 376.7 | 616.5 | 69046.4 |
| Entry | Solvent | Conversion of 1 (%) | Selectivity to 2 (%) | Selectivity to 3 (%) |
|---|---|---|---|---|
| 1 | Nitromethane | 96.8 | 2.7 | 97.3 |
| 2 | Ethanol | 28.4 | 11.7 | 88.3 |
| 3 | Dichloromethane | 49.0 | 6.0 | 94.0 |
| 4 | Toluene | 41.6 | 5.0 | 95.0 |
| 5 | DMF | 5.9 | 15.8 | 84.2 |
| 6 | Water | 16.7 | 32.3 | 67.7 |
| 7 | THF | 27.7 | 21.8 | 78.2 |
| Entry | Catalysts b | Conversion of 1 (%) | Selectivity to 2 (%) | Selectivity to 3 (%) |
|---|---|---|---|---|
| 1 | SA/LDH-Mg2Al-80 | 96.8 | 2.7 | 97.3 |
| 2 | SA/LDH-Mg2Al-105 | 59.3 | 30.9 | 69.1 |
| 3 | SA/LDH-Mg2Al-150 | 51.9 | 28.1 | 71.9 |
| 4 | SA/LDH-Mg5Al2-80 | 98.2 | 21.4 | 78.6 |
| 5 | SA/LDH-Mg3Al-80 | 92.7 | 12.8 | 87.2 |
| 6 | SA | Trace | — | Trace |
| 7 | LDH-Mg2Al-80 | 75.9 | 13.6 | 86.4 |
| 8 | None | Trace | — | Trace |
| Entry | Catalysts | Conversion of 1 (%) | Yield of 2 (%) a | Yield of 3 (%) a | Temp. (°C) | Time (h) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | SA/LDH-Mg2Al-80 | 96.8 | 2.6 | 94.2 | 80 | 6 | — |
| 2 | SA/LDH-Mg5Al2-80 | 98.2 | 21.0 | 77.2 | 80 | 6 | — |
| 3 | CaO | 60.1 | — | — | 102 | 12 | [58] |
| 4 | MgO | 3.0 | 2.1 | — | 65 | 6 | [59] |
| 5 | Al2O3 | — | 37 b | — | — | 12 | [60] |
| 6 | MgO | — | 51 b | — | — | 8 | [60] |
| 7 | Modified Mg-Al LDH | — | 95 b | — | — | 0.5 | [60] |
| 8 | Tb-MOF-NH2 | 87.0 | — | — | 90 | 24 | [61] |
| 9 | TMAOH intercalated layered silicate | 55.0 | 41.8 | 3.74 | R.T. | 6 | [62] |
| 10 | silica-alumina-NH2 | — | — | 99.0 | 100 | 6 | [63] |
| 11 | SiO2-NH2 | — | — | 37.0 | 100 | 6 | [63] |
| 12 | Si-Zr-Ti/PAI-HFs-NH2 | 80 | 10.4 | 69.6 | 50 | 4 | [64] |
| 13 | Mg-NHC | — | 74 | — | 60 | 12 | [65] |
| 14 | Diamino modified- LDH/silicate composite | 96 | 0.48 | 95.52 | 70 | 2 | [66] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Wang, Y.; Wang, X.; Feng, X.; Ye, X.; Fu, J. Small-Sized Mg–Al LDH Nanosheets Supported on Silica Aerogel with Large Pore Channels: Textural Properties and Basic Catalytic Performance after Activation. Nanomaterials 2018, 8, 113. https://doi.org/10.3390/nano8020113
Wang L, Wang Y, Wang X, Feng X, Ye X, Fu J. Small-Sized Mg–Al LDH Nanosheets Supported on Silica Aerogel with Large Pore Channels: Textural Properties and Basic Catalytic Performance after Activation. Nanomaterials. 2018; 8(2):113. https://doi.org/10.3390/nano8020113
Chicago/Turabian StyleWang, Lijun, Yusen Wang, Xiaoxia Wang, Xiaolan Feng, Xiao Ye, and Jie Fu. 2018. "Small-Sized Mg–Al LDH Nanosheets Supported on Silica Aerogel with Large Pore Channels: Textural Properties and Basic Catalytic Performance after Activation" Nanomaterials 8, no. 2: 113. https://doi.org/10.3390/nano8020113
APA StyleWang, L., Wang, Y., Wang, X., Feng, X., Ye, X., & Fu, J. (2018). Small-Sized Mg–Al LDH Nanosheets Supported on Silica Aerogel with Large Pore Channels: Textural Properties and Basic Catalytic Performance after Activation. Nanomaterials, 8(2), 113. https://doi.org/10.3390/nano8020113